This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
This article has been viewed 30,426 times.
When harmful gases contained in air pollution react with moisture in the air, acid rain can result. Ordinary rain is naturally acidic, with a pH between 5.0 and 5.5.[1] Acid rain generally has a lower and thus more acidic pH in the range of 4.0-4.4. This highly acidic rain is dangerous to plants, animals, insects, and other organisms around the world. You can measure the acidity of your own rain by collecting it and then using one of a variety of pH indicators such as pH paper, a pH meter, or your own homemade pH solution made from red cabbage.
Steps
Testing the Acidity with pH Paper
-
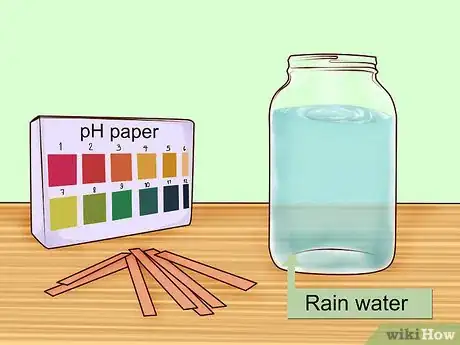
1Gather the necessary materials. For this experiment, you will need to collect some rain water and get some strips of pH paper. pH paper is a special type of paper that changes color based on how acidic or basic a solution is. The paper comes with a separate color chart that allows you to identify the pH according to the color.[2]
- pH paper can be purchased at gardening or pool stores.
-
2Collect rain in a clean container. To test the acidity of your rainwater, you want to obtain a sample that is free from contaminants. Place a clean jar or bucket outside while it is raining. Make sure the bucket is at least two meters (six-and-a-half feet) above the ground to prevent contamination from dirt and dust.[3]
- Place the bucket on a table or hang it above the ground.
- Contaminants can alter the pH of the collected rain.
Advertisement -
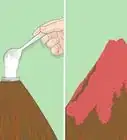
3Dip the pH paper into the collected rain. Take a single strip of pH paper from the stack and dip it into the rainwater for about two seconds. The color should change immediately and then you should proceed to the next step.[4]
- If you let the strip sit for too long without looking at the chart, the color might change.
- Try the test at least three times and see if they all come out the same color. If the strips do not come out the same color, the strips may be expired and you’ll need to buy new, fresh strips.
-
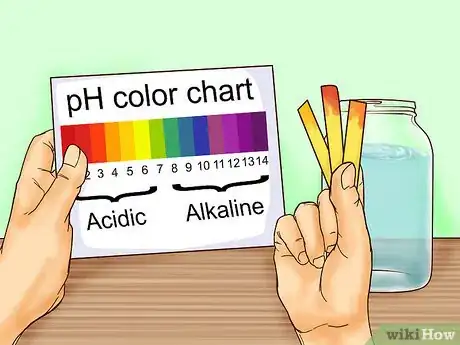
4Compare the color paper to the pH chart. As soon as the color changes on the strip, compare it to the associated chart. Find the color that is the closest match to what you see on the strip. The pH written next to that color is the pH of the collected rainwater.
- The color comparison must happen immediately because the color will fade with time.
- Normal rainwater is slightly acidic with a pH between 5.0 and 5.5. Acid rain usually has a pH of around 4.0 but anything in the range of 4.2-4.4 is considered acidic.[5]
Testing the Acidity with a Homemade pH Indicator
-
1Gather the necessary materials. To make your own pH indicator, you will need half a head of red cabbage, a knife, water, a stove, and a medium saucepan. Red cabbage contains a chemical called anthocyanin that changes color based on acidity. By boiling the cabbage, you can make a pH indicator from this chemical and test the acidity of your rain.[6]
- Adult supervision is recommended for this because you will need to handle a hot stove and a sharp knife.
-
2Chop up some cabbage. You want about 2-3 cups of red cabbage cut into small pieces. If you are young, have an adult help you with this step to avoid potential injury. The exact amount of cabbage is not important, so just cut up half of the head of cabbage.[7]
-
3Boil the cabbage. Place the chopped cabbage into the saucepan and add enough water to cover the cabbage. Put the saucepan on the stove and bring the water to a full boil. Once the solution boils, turn the heat off and let the water cool to room temperature.[8]
- Have a parent help with this step to avoid injury when using the stove and working with boiling water.
- You should notice that the water is now purple.
-
4Strain out the cabbage and save the water. Pour the cabbage and water mixture through a strainer into a bowl. The purple water (the indicator) is currently neutral because the water from your tap is neutral. In the presence of an acid, the indicator turns pink. In the presence of a base, the indicator turns blue.[9]
- You can refrigerate the solution until you need it.
-
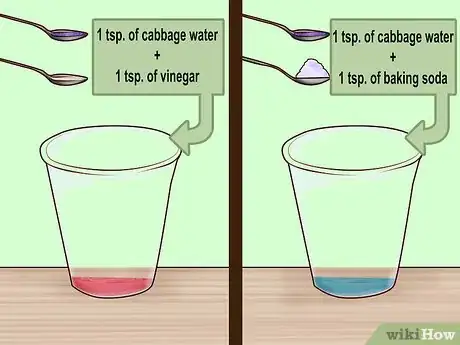
5Test the indicator. To make sure the indicator is working properly, you can test it using baking soda and vinegar. Put 1 teaspoon (~5 mL) of the cabbage water into two small, clear glasses. Add a teaspoon of vinegar to one glass and a teaspoon of baking soda to the other.[10]
- Vinegar is acidic, so the water will turn pink.
- Baking soda is basic, so the water will turn blue.
-
6Test the acidity of your rain. Collect some rainwater in a clean, plastic container. Pour 1 teaspoon of cabbage water into a small, clean glass. Add a teaspoon (~5 mL) of the collected rainwater to the cabbage water. The water should turn pink because all rain is slightly acidic. The deeper the pink, the higher the acidity in your area.[11]
- This method will not tell you the exact pH of the rain, but will give you an idea of how acidic it is. If you are traveling, you can compare rain from different areas such as different cities, state or province, or even countries and see if the deepness of the pink changes.
Using a pH Meter
-
1Gather the necessary materials. Another method to measure the acidity of a solution is to use a pH meter. This is a specialized piece of laboratory equipment that is expensive and you may not have access to one. If you do have one available, you can use it to determine the exact pH of a solution.[12] For this experiment, you will need:
- Rainwater
- A pH meter
- Calibration buffers (one with pH 7.0 and one with pH 2.0)
-
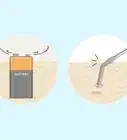
2Collect some rainwater. During a rainstorm, place a clean container outside at a height of at least two meters (six-and-a-half feet). Placing the bucket up high prevents dust and dirt from getting into the rainwater. These contaminants can affect the pH of the water.[13]
- Collect at least one cup (250 mL) of rainwater for measurement.
-
3Calibrate the pH meter. Before using a pH meter, it must be calibrated so that it gives you the correct reading of your sample. Each meter is different but the general calibration process is the same. First, rinse the electrode of the meter with water and then place it into a buffer with a known pH of 7.0. Set the readout display to 7.0 so the meter knows that this is what a neutral pH is.
- Repeat this process with another buffer that is acidic (around pH 2.0).
- Some meters may also require that you calibrate with a buffer that is also basic (around pH 10.0).
- Always rinse the electrode between solutions.
-
4Measure the acidity of rainwater. Rinse the electrode with distilled water before use. After calibration, you can place the electrode in the rainwater. Once the reading has stabilized on the meter, the display is the pH of the rain.
- Remember, normal rain has a pH between 5.0 and 5.5. Acid rain is usually in the range of 4.0-4.4.[14]
Community Q&A
-
QuestionHow will I know if rain is acid rain?
 Community AnswerYou would have to check the pH of the rain (the pH is an indicator if something is acidic, neutral or alkaline). Collect the rainwater and use a pH indicator. If the pH is less than 7, the rain water is acidic.
Community AnswerYou would have to check the pH of the rain (the pH is an indicator if something is acidic, neutral or alkaline). Collect the rainwater and use a pH indicator. If the pH is less than 7, the rain water is acidic.
Warnings
- Stoves are always hot! Always have adult supervision when you use a stove.⧼thumbs_response⧽
- Knives are sharp! Don't cut yourself! Have adult supervision when handling knives.⧼thumbs_response⧽
Things You’ll Need
pH Paper
- Small bucket
- Rain water
- pH test strips
Homemade Indicator
- Half head of red cabbage
- Knife
- Tap water
- Stove
- Medium saucepan
- Baking soda
- Vinegar
- Rain water
pH Meter
- Small bucket
- Rain water
- pH meter
- Calibration buffers (pH 10.0 and pH 2.0)
References
- ↑ https://www3.epa.gov/acidrain/education/site_students/phscale.html
- ↑ http://scienceline.ucsb.edu/getkey.php?key=49
- ↑ http://scienceline.ucsb.edu/getkey.php?key=49
- ↑ http://scienceline.ucsb.edu/getkey.php?key=49
- ↑ https://www3.epa.gov/acidrain/education/site_students/phscale.html
- ↑ http://www.sciencekiddo.com/red-cabbage-ph-indicator/
- ↑ http://www.sciencekiddo.com/red-cabbage-ph-indicator/
- ↑ http://www.sciencekiddo.com/red-cabbage-ph-indicator/
- ↑ http://www.sciencekiddo.com/red-cabbage-ph-indicator/














-Step-15.webp)