This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
This article has been viewed 62,702 times.
Gel is a state that is not quite solid or liquid, it is something in between. On its own, water has three states: solid, liquid, and vapor. With the addition of sodium polyacrylate or agar, it is possible to turn water into a fourth state: gel. The sodium polyacrylate method should be performed in a lab, but the edible method can be performed in the comforts of your kitchen.
Steps
Using Sodium Polyacrylate
-
1Get some sodium polyacrylate. Sodium polyacrylate is an absorbent polymer that can absorb up to 400 times its weight. It is found in many absorbent products, such as diapers, and you can buy it online. It is occasionally sold as "water gel powder," "slush powder," or "instant solid powder."[1]
-
2Put on some safety goggles. You will be working with chemicals, so putting on a pair of safety goggles is a must.[2] Polymers absorb water, so this is not something that you want to get in your eyes.[3]Advertisement
-
3Measure 5 to 7 grams (0.18 to 0.25 oz) of sodium polyacrylate into a glass container. This may seem like a tiny amount, but you don't need much to turn water into gel. The polymer will swell up once you add the water.[4]
-
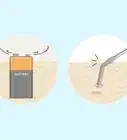
4Add 300 milliliters of water. Try to pour the water quickly, and from about 12 inches (30 cm) or even higher; this will cause the water to mix into the polymer better.[5]
- If you have access to it, use deionized water for best results.
-
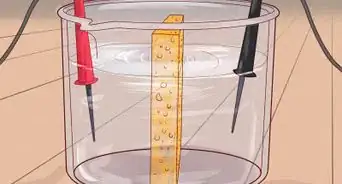
5Watch as the water turns into gel. Sodium polyacrylate has both carboxylate anion protons and sodium cation protons. Since water is attracted to sodium ions, it enters the polymer through osmosis. This causes the sodium carboxylate to ionize and the polymer to swell. Meanwhile, the anionic carboxylates repel each other, causing further swelling, which traps the water, and results in a gel.[6]
-
6Stir some salt into the to reverse the process. This is not a required part of the experiment, but it is an interesting follow-up. NaCL (sodium chloride, also known as table salt) increases the number of electrolytes (ions) in the water. This prevents the carboxylates from repelling each other and causes the polymer to contract and expel the water.[7]
- Start with about 5 to 7 g (1 to 1.25 teaspoons) of salt, then add more as needed. Keep adding salt and stirring until the gel turns back to water.
- Use a glass stirring rod or stick for this.
-
7Discard the gel. If your lab has a waste bin for chemicals, you can pour the gel in there. You can also stir salt into it, as indicated in the previous step, to return it to a liquid state, then pour it down the drain.[8]
Making Edible Water Gel
-
1Measure out 3⅓ cups (80 grams) of agar powder into a large saucepan. Make sure that you are using agar powder and not agar flakes. Also, make sure that the saucepan is big enough to hold at least 2 liters (0.53 US gal) of water.[9]
-
2Gradually stir 8 cups (2 liters) of water into the saucepan. Do not add the water all at once. Instead, start with just enough water to turn the powder into a paste. Then add the rest of the water in about four increments, stirring between each one.[10]
-
3Boil the agar water. Set the saucepan on the stove, and turn the heat up to medium. Cook the agar water for 3 to 5 minutes, or until it reaches 176°F (80°C).
-
4Chill the agar water in an ice bath. Fill a larger container (ie: a sink) with cold water, then add some ice. Set the saucepan into the water, then stir the agar water until it turns cold and clear.[11]
- The water level in the ice bath needs to reach the agar water level in the saucepan.
-
5Pour the agar into your desired mold(s). You can use silicone baking or ice cube trays, regular ice cube trays, or even candy making molds. You can even use regular jelly molds.
-
6Refrigerate the agar water for 2 hours. As the agar water continues to cool down, it will solidify into a gel. It will be slightly foggy, but still clear enough to see through.[12]
-
7De-mold and serve the gel. The gel won't taste like much, so it would be a good idea to pour some sugar water or simple syrup over it before digging in. You can even use honey or a flavored simple syrup instead.[13]
Community Q&A
-
QuestionAt what temperature will it return to a liquid?
 NyamburaCommunity AnswerIt depends on what substance you are talking about. Water vapour turns to a gas at 100 degrees Celsius and it turns to a liquid below that temperature level. Some substances do not turn into liquids at all whether you heat it or cool it a good example is iodine, this is called sublimation.
NyamburaCommunity AnswerIt depends on what substance you are talking about. Water vapour turns to a gas at 100 degrees Celsius and it turns to a liquid below that temperature level. Some substances do not turn into liquids at all whether you heat it or cool it a good example is iodine, this is called sublimation. -
QuestionDoes agar gel eventually change to liquid state; when it is kept in room temperature?
 HannahCommunity AnswerNo, it will not change to liquid state. The only way to liquefy agar is to heat it up to about 95°C (about 203° Fahrenheit).
HannahCommunity AnswerNo, it will not change to liquid state. The only way to liquefy agar is to heat it up to about 95°C (about 203° Fahrenheit). -
QuestionIs the water gel safe to apply to your skin?
 Cros SaintCommunity AnswerFor the first method, no, it is not safe. For the second method, it is likely safe as it is just agar and water, unless you have skin sensitivity to agar.
Cros SaintCommunity AnswerFor the first method, no, it is not safe. For the second method, it is likely safe as it is just agar and water, unless you have skin sensitivity to agar.
Warnings
Things You'll Need
Using Sodium Polyacrylate
- Sodium polyacrylate
- Water (preferably deionized)
- NaCl (sodium chloride/table salt)
- Glass container or beaker
- Glass stir rod
- Scale
- Safety goggles
Making Edible Water Gel
- 3⅓ cups (80 grams) agar powder
- 8 cups (2 liters) water
- Large saucepan
- Rubber spatula
- Plastic or silicone molds
References
- ↑ https://www.youtube.com/watch?v=sqgq7dIeVsE
- ↑ https://www.cmu.edu/gelfand/lgc-educational-media/polymers/polymer-and-absorption/super-absorb-powder.html
- ↑ https://www.chemicalbook.com/ChemicalProductProperty_EN_CB9741267.htm
- ↑ https://www.cmu.edu/gelfand/lgc-educational-media/polymers/polymer-and-absorption/super-absorb-powder.html
- ↑ https://sites.lib.jmu.edu/chemdemos/2011/06/14/make-water-a-gel/
- ↑ https://sites.lib.jmu.edu/chemdemos/2011/06/14/make-water-a-gel/
- ↑ https://sites.lib.jmu.edu/chemdemos/2011/06/14/make-water-a-gel/
- ↑ https://sites.lib.jmu.edu/chemdemos/2011/06/14/make-water-a-gel/
- ↑ https://www.youtube.com/watch?v=gSDuWkMnT0Y
- ↑ https://www.youtube.com/watch?v=gSDuWkMnT0Y
- ↑ https://www.youtube.com/watch?v=gSDuWkMnT0Y
- ↑ https://www.youtube.com/watch?v=gSDuWkMnT0Y
- ↑ https://www.youtube.com/watch?v=gSDuWkMnT0Y
- ↑ https://www.poison.org/articles/whats-inside-ice-packs-201
- ↑ https://www.poison.org/articles/whats-inside-ice-packs-201















-Step-15.webp)