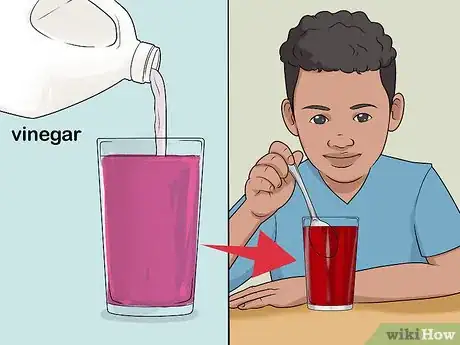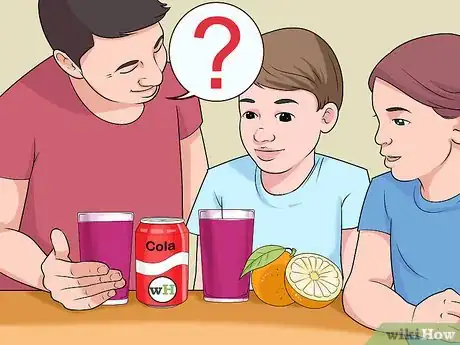This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
There are 7 references cited in this article, which can be found at the bottom of the page.
This article has been viewed 184,788 times.
If you’ve got a little chemist in your house, teaching them about acids and bases is a fun and fascinating project. Since acids and bases are everyday substances, it’s easy to make the concepts relatable. You can discuss things that help kids understand acids and bases (such as the pH scale), but it’s also possible to make your very own indicator at home. Use this indicator to have kids test out various substances to see if they are acidic or basic. Get creative and have some fun experimenting!
Steps
Explaining the Properties of Acids and Bases
-
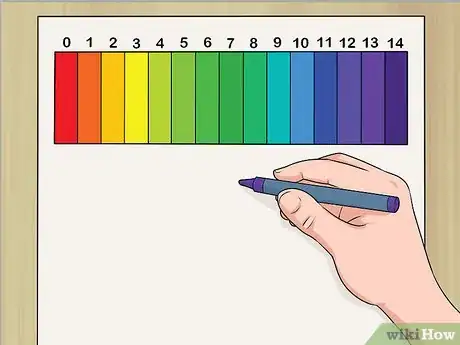
1Draw out the pH scale. Get some paper and markers or crayons. Draw a long, thin, vertical rectangle and draw lines to divide it into 14 sections. Have kids color in each section a different color. Try to create a scale of gradually shifting colors -- for instance, start with light yellow in the bottom section, then move through yellow-orange, orange, red-orange, red, violet, purple, indigo, blue, blue-green, etc.
-
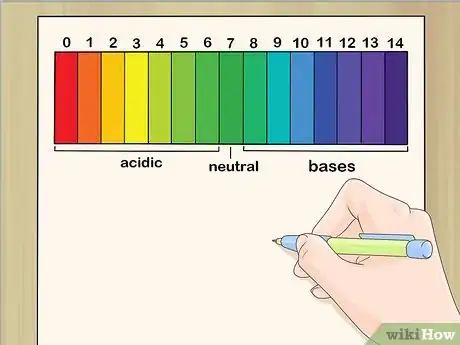
2Label the pH scale. Have the kids label each section of the scale with a number in consecutive order, with 0 at the bottom and 14 at the top. Write “Acids” near the bottom and “Bases” at the top. Explain that numbers 0-6.9 apply to acids, 7 is neutral, and 7.1-14 refer to bases.[1]Advertisement
-
3Talk about common acids and bases. Explain that acids and bases are found all over the place. For instance, bodies use acids to help digest food, and many cleaning products contain bases. Ask the kids to name some common substances and guess if they are acidic or basic.[2]
- You can mention that acidic substances, like orange juice or tomatoes, taste sour. Bases, like baking soda or soap, are bitter.
- This is also a good time to explain that some acids and bases are very strong and can be harmful. Battery acid and ammonia (a base) are two dangerous substances that may be encountered at home, for instance.
- Another activity might be to have the children draw or write the mane of some common acids and bases and then identify where they fall on the acid/base scale.
-
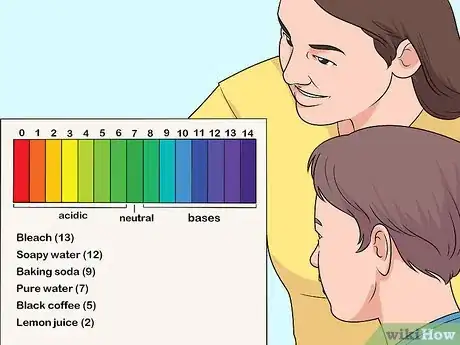
4Explain what the scale shows. Tell the kids that some substances are acidic and some are basic, and that the pH scale helps people determine how strong those substances are. Point out that many common substances are acids and bases, and mark them on the scale. Common substances and their pH levels include:[3]
- Bleach (13)
- Soapy water (12)
- Baking soda (9)
- Pure water (7)
- Black coffee (5)
- Lemon juice (2)
-
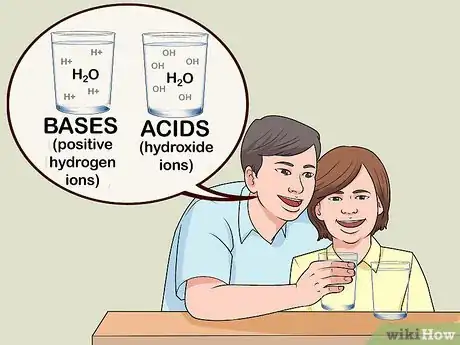
5Discuss the chemistry behind acids and bases. If the kids are more advanced or have some knowledge of chemistry, explain that bases produce negative hydroxide ions (OH-) and acids produce positive hydrogen ions (H+). The greater the concentration of H+ ions, the stronger the acid (and vice versa).[4]
- If the kids know a bit about atoms and molecules, but are new to the concept of ions, just explain that they are particles with a particular charge (positive or negative).
- You can also mention that acids and bases neutralize each other because mixing them changes the relative concentrations of positive and negative ions. So, if you add baking soda (a base) to vinegar (an acid), the mixture’s pH will move closer to 7 (the neutral point on the pH scale).
Experimenting with an Indicator
-
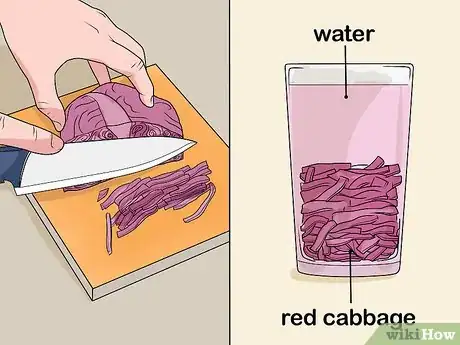
1Make some red cabbage juice. Take a head of red cabbage and slice it into thin strips. Let it simmer for 30 minutes in enough water to cover the strips. Strain the juice through a colander and store it in another pot. Let it cool.[5]
-
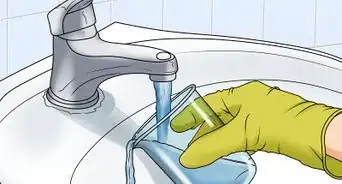
2Pour some of the juice into clear cups. Explain that the red cabbage juice is called an “indicator.” This means that it will help you figure out if a substance is an acid or a base. Take some of the juice and pour it into several clear cups. Put the rest to the side for now.[6]
- It doesn't matter how much you pour in each cup. A few ounces will be fine, and should leave you enough experiment with several substances.
- Use as many cups as you have substances to test. For instance, if you want to test milk, tomato juice, and soy sauce, use three cups.
-
3Add baking soda to the solution. Take a spoonful of baking soda and pour it into one of the glasses. Have a kid stir until the soda begins to dissolve. The solution will turn from red to blue or purplish.[7]
- Explain the indicator solution changes this color because baking soda is a base.
-
4Pour vinegar into the solution. Take some ordinary white vinegar and pour it into the same glass as the baking soda. Ask a kid to stir the solution. It will turn red again before your eyes![8]
- Explain this is because the acidic vinegar changes the pH of the solution by neutralizing the base (baking soda).
-
5Try adding different substances to the indicator solution. Practice stirring different substances into cups of the solution. Drinks like cola, lemon juice, or milk work well. Before you try each substance, ask the kids if they think the solution will turn bluish (meaning it’s a base) or a deeper red (meaning it’s an acid).[9]
- To help the kids decide, ask them to think about whether or not the substance tastes sour (acid) or bitter (base).
Community Q&A
-
QuestionWhat are 3 properties of acids and bases?
 Community AnswerAcids taste sour, feel sticky, and turns litmus paper to red. On the other hand, bases taste bitter, feel slippery, and turns litmus paper to blue.
Community AnswerAcids taste sour, feel sticky, and turns litmus paper to red. On the other hand, bases taste bitter, feel slippery, and turns litmus paper to blue. -
QuestionHow do acids and alkali react together?
 SUDEEKSHA BHATTACHARYYACommunity AnswerAcid-Base reaction is a Neutralization reaction. The more reactive metal in a compound displaces the less reactive metal in another compound thus, resulting in the formation of two new compounds. Now, let's take an example: HCl(aq) + NaOH(aq)----> NaCl(aq) + H2O(l). Here, HCl is a dilute acid and NaOH is an alkali (Base dissolved in water). Na is more reactive than H and thus displaces it and forms NaCl(aq). On the other hand, H+ combines with OH- and forms H2O. In general, Acid-Base/alkali reaction can be written as: Acid + Base ----> Salt + Water.
SUDEEKSHA BHATTACHARYYACommunity AnswerAcid-Base reaction is a Neutralization reaction. The more reactive metal in a compound displaces the less reactive metal in another compound thus, resulting in the formation of two new compounds. Now, let's take an example: HCl(aq) + NaOH(aq)----> NaCl(aq) + H2O(l). Here, HCl is a dilute acid and NaOH is an alkali (Base dissolved in water). Na is more reactive than H and thus displaces it and forms NaCl(aq). On the other hand, H+ combines with OH- and forms H2O. In general, Acid-Base/alkali reaction can be written as: Acid + Base ----> Salt + Water. -
QuestionWhat are some examples for indicators when explaining acids and bases?
 HanCommunity AnswerCertain plants, like cabbage, blueberries and poppy petals, contain compounds (anthocyanins) that function as pH indicators . Chop/smash the plant and put them in a bit of water, so the compounds get released into the water. You can the use this solution as a crude pH indicator. Some common indicators used in the lab are universal indicator, phenolphthalein and bromothymol blue.
HanCommunity AnswerCertain plants, like cabbage, blueberries and poppy petals, contain compounds (anthocyanins) that function as pH indicators . Chop/smash the plant and put them in a bit of water, so the compounds get released into the water. You can the use this solution as a crude pH indicator. Some common indicators used in the lab are universal indicator, phenolphthalein and bromothymol blue.
Things You'll Need
- Red cabbage
- Two pans
- Colander
- Several clear cups
- Baking soda
- Spoon
- Vinegar
- Other substances (like cola, lemon juice, or milk)
References
- ↑ http://www.ducksters.com/science/acids_and_bases.php
- ↑ http://www.ducksters.com/science/acids_and_bases.php
- ↑ http://www.ducksters.com/science/acids_and_bases.php
- ↑ https://www.khanacademy.org/science/biology/water-acids-and-bases/acids-bases-and-ph/a/acids-bases-ph-and-bufffers
- ↑ https://www.teachengineering.org/activities/view/wst_environmental_lesson02_activity3
- ↑ https://ctsciencecenter.org/blog/science-at-play-red-cabbage-juice-indicator/
- ↑ https://www.acs.org/education/outreach/activities/red-cabbage-indicator.html
- ↑ https://whyy.pbslearningmedia.org/resource/phy03.sci.phys.matter.zcabbage/acids-and-bases-cabbage-juice-indicator/#.WeAVUtEpDIU
- ↑ https://whyy.pbslearningmedia.org/resource/phy03.sci.phys.matter.zcabbage/acids-and-bases-cabbage-juice-indicator/#.WeAVUtEpDIU
About This Article
Explaining acids and bases to kids can be a fun way to introduce them to chemistry and pH indicators. Before you experiment, draw out the 14-section pH scale and have your kids help you color it in and label the different sections. Once you finish the chart, it’s helpful to point out some common substances that are acids or bases. For example, bleach is a number 13 base and lemon juice is a number 2 acid. Once your kids understand the basic concept, try making a liquid indicator so you can experiment. All you have to do is boil chopped up red cabbage for 30 minutes, then strain the cabbage juice into a cup. Now, you can add substances to the cup to help determine if they’re an acid, neutral, or base. For example, when you add baking soda to the indicator, it will turn purple, indicating baking soda is a base. Then, if you add vinegar, the cabbage juice will turn red again, meaning the vinegar is an acid and it neutralized the base. To learn more ways to experiment with acids and bases, read on!