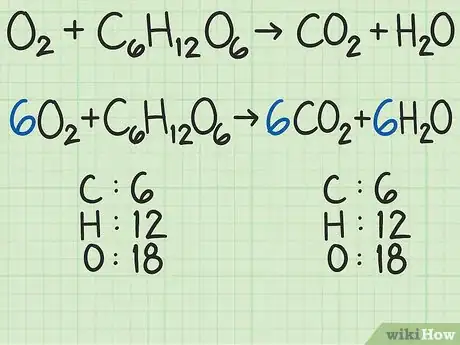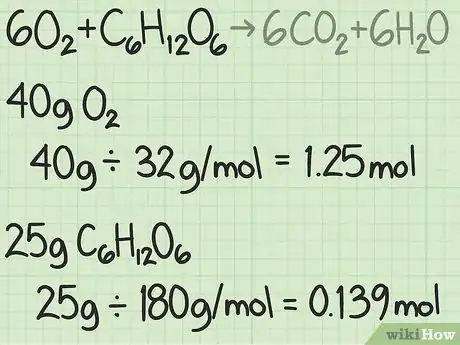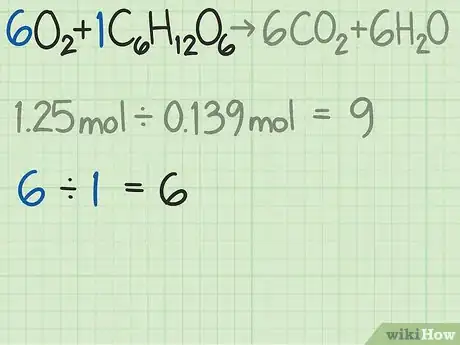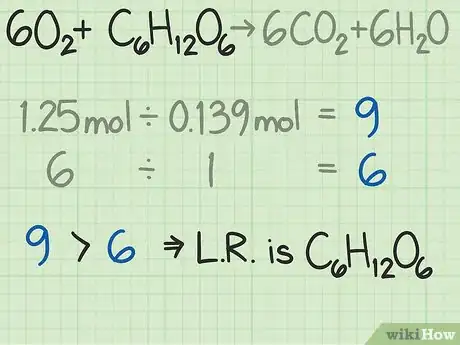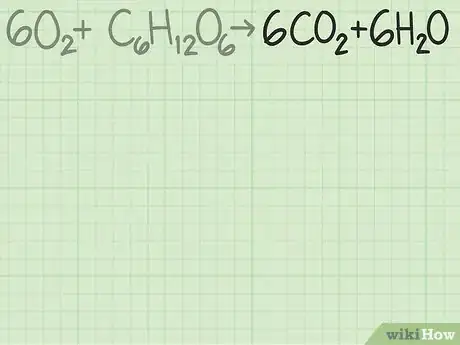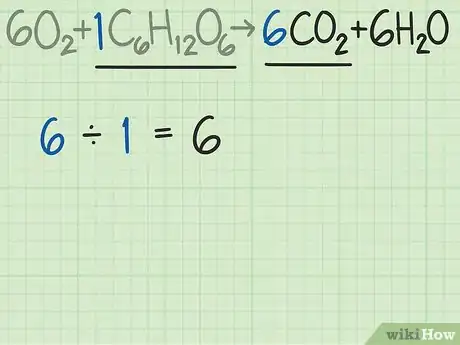This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
This article has been viewed 955,936 times.
The theoretical yield is a term used in chemistry to describe the maximum amount of product that you expect a chemical reaction could create. You need to begin with a [Balance-Chemical-Equations|balanced chemical equation]] and define the limiting reactant. When you measure the amount of that reactant that you will be using, you can calculate the amount of product. This is the theoretical yield of the equation. To learn how to calculate theoretical yield using the theoretical yield formula, keep reading!
Steps
Finding the Limiting Reactant
-
1Start with a balanced chemical equation. A chemical equation is like a recipe. It shows the reactants (on the left side) reacting to form products (on the right side). A properly balanced equation will show the same number of atoms going into the equation as reactants as you have coming out in the form of products.[1]
- For example, consider the simple equation → . There are two atoms of hydrogen on both the left and right. But there are two atoms of oxygen going in as a reactant and only one atom in the product on the right.
- To balance, double the product, to get → .
- Check the balance. This change has corrected the oxygen, which now has two atoms on both sides. But you now have two atoms of hydrogen on the left with four atoms of hydrogen on the right.
- Double the hydrogen in the reactant. This will adjust the equation to → . This change now has 4 atoms of hydrogen on both sides, and two atoms of oxygen. The equation is balanced.
- As a more complicated example, oxygen and glucose can react to form carbon dioxide and water: →
In this equation, each side has exactly 6 carbon (C) atoms, 12 hydrogen (H) atoms, and 18 oxygen (O) atoms. The equation is balanced.
-
2Calculate the molar mass of each reactant. Using the periodic table or some other reference, look up the molar mass of each atom in each compound. Add them together to find the molar mass of each compound of reactant. Do this for a single molecule of the compound.[2] Consider again the equation of converting oxygen and glucose into carbon dioxide and water: →
- For this example, one molecule of oxygen () contains two oxygen atoms.
- The molar mass of one atom of oxygen is about 16 g/mol. If necessary, you can find more precise values.)
- 2 oxygen atoms x 16 g/mol per atom = 32 g/mol of .
- The other reactant, glucose () has a molar mass of (6 atoms C x 12 g C/mol) + (12 atoms H x 1 g H/mol) + (6 atoms O x 16 g O/mol) = 180 g/mol.
Advertisement -
3Convert the amount of each reactant from grams to moles. For an actual experiment, you will know the mass in grams of each reactant that you are using. Divide this value by that compound's molar mass to convert the amount to moles.[3]
- For example, suppose you begin with 40 grams of oxygen and 25 grams of glucose.
- 40 g / (32 g/mol) = 1.25 moles of oxygen.
- 25g / (180 g/mol) = about 0.139 moles of glucose.
-
4Determine the molar ratio of the reactants. A mole is a tool used in chemistry to count molecules, based on their mass. By determining the number of moles of both oxygen and glucose, you know how many molecules of each you are starting with. To find the ratio between the two, divide the number of moles of one reactant by the number of moles of the other.[4]
- In this example, you are starting with 1.25 moles of oxygen and 0.139 moles of glucose. Thus, the ratio of oxygen to glucose molecules is 1.25 / 0.139 = 9.0. This ratio means that you have 9 times as many molecules of oxygen as you have of glucose.
-
5Find the ideal ratio for the reaction. Look at the balanced equation for the reaction. The coefficients in front of each molecule tell you the ratio of the molecules that you need for the reaction to occur. If you use exactly the ratio given by the formula, then both reactants should be used equally.[5]
- For this reaction, the reactants are given as . The coefficients indicate that you need 6 oxygen molecules for every 1 glucose molecule. The ideal ratio for this reaction is 6 oxygen / 1 glucose = 6.0.
-
6Compare the ratios to find the limiting reactant. In most chemical reactions, one of the reactants will be used up before the others. The one that gets used up first is called the limiting reactant. This limiting reactant determines how long the chemical reaction can take place and the theoretical yield you can expect. Compare the two ratios you calculated to identify the limiting reactant:[6]
- In this example, you are beginning with 9 times as much oxygen as glucose, when measured by number of moles. The formula tells you that your ideal ratio is 6 times as much oxygen as glucose. Therefore, you have more oxygen than required. Thus, the other reactant, glucose in this case, is the limiting reactant.
Determining Theoretical Yield
-
1Review the reaction to find the desired product. The right side of a chemical equation shows the products created by the reaction. The coefficients of each product, if the reaction is balanced, tells you the amount to expect, in molecular ratios. Each product has a theoretical yield, meaning the amount of product you would expect to get if the reaction is perfectly efficient.[7]
- Continuing the example above, you are analyzing the reaction → . The two products shown on the right are carbon dioxide and water.
- You can begin with either product to calculate theoretical yield. In some cases, you may be concerned only with one product or the other. If so, that is the one you would start with.
-
2Write down the number of moles of your limiting reactant. You must always compare moles of reactant to moles of product. If you try to compare the mass of each, you will not reach the correct results.[8]
- In the example above, glucose is the limiting reactant. The molar mass calculations found that the initial 25g of glucose are equal to 0.139 moles of glucose.
-
3Compare the ratio of molecules in product and reactant. Return to the balanced equation. Divide the number of molecules of your desired product by the number of molecules of your limiting reactant.[9]
- The balanced equation for this example is → . This equation tells you that you expect 6 molecules of the desired product, carbon dioxide (), compared to 1 molecule of glucose ().
- The ratio of carbon dioxide to glucose is 6/1 = 6. In other words, this reaction can produce 6 molecules of carbon dioxide from one molecule of glucose.
-
4Multiply the ratio by the limiting reactant's quantity in moles. The answer is the theoretical yield, in moles, of the desired product.[10]
- In this example, the 25g of glucose equate to 0.139 moles of glucose. The ratio of carbon dioxide to glucose is 6:1. You expect to create six times as many moles of carbon dioxide as you have of glucose to begin with.
- The theoretical yield of carbon dioxide is (0.139 moles glucose) x (6 moles carbon dioxide / mole glucose) = 0.834 moles carbon dioxide.
-
5Convert the result to grams. This is the reverse of your earlier step of calculating the number of moles or reactant. When you know the number of moles that you expect, you will multiply by the molar mass of the product to find the theoretical yield in grams.[11]
- In this example, the molar mass of CO2 is about 44 g/mol. (Carbon's molar mass is ~12 g/mol and oxygen's is ~16 g/mol, so the total is 12 + 16 + 16 = 44.)
- Multiply 0.834 moles CO2 x 44 g/mol CO2 = ~36.7 grams. The theoretical yield of the experiment is 36.7 grams of CO2.
-
6Repeat the calculation for the other product if desired. In many experiments, you may only be concerned with the yield of one product. If you wish to find the theoretical yield of both products, just repeat the process.
- In this example, the second product is water, . According to the balanced equation, you expect 6 molecules of water to come from 1 molecule of glucose. This is a ratio of 6:1. Therefore, beginning with 0.139 moles of glucose should result in 0.834 moles of water.
- Multiply the number of moles of water by the molar mass of water. The molar mass is 2 + 16 = 18 g/mol. Multiplying by the product, this results in 0.834 moles H2O x 18 g/mol H2O = ~15 grams. The theoretical yield of water for this experiment is 15 grams.
Community Q&A
-
QuestionDoesn't one molecule of glucose produce six molecules of water, not one?
 Community AnswerYes. One molecule of glucose plus six molecules of oxygen = six molecules of water plus six molecules of carbon dioxide.
Community AnswerYes. One molecule of glucose plus six molecules of oxygen = six molecules of water plus six molecules of carbon dioxide. -
QuestionWhat should I do if there is more than one reactant?
 Community AnswerFind out which of the reactants is the "limiting" reactant and use that to calculate the theoretical yield. This can be done using Part 1 of this article.
Community AnswerFind out which of the reactants is the "limiting" reactant and use that to calculate the theoretical yield. This can be done using Part 1 of this article. -
QuestionWhat should I do if the reactants have the same number of moles?
 HannahCommunity AnswerThat's not a problem! It only means that the molar ratio of your reactants is 1. In the next step, you need to compare it to the ideal molar ratio from your chemical equation to find the limiting reactant and continue as described in the article.
HannahCommunity AnswerThat's not a problem! It only means that the molar ratio of your reactants is 1. In the next step, you need to compare it to the ideal molar ratio from your chemical equation to find the limiting reactant and continue as described in the article.
References
- ↑ http://www.chemteam.info/Equations/Balance-Equation.html
- ↑ https://www.khanacademy.org/science/ap-chemistry-beta/x2eef969c74e0d802:chemical-reactions/x2eef969c74e0d802:stoichiometry/a/limiting-reagents-and-percent-yield
- ↑ http://www.chemteam.info/Stoichiometry/Limiting-Reagent.html
- ↑ http://www.chemteam.info/Equations/Balance-Equation.html
- ↑ http://www.chemteam.info/Equations/Balance-Equation.html
- ↑ https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/limiting-reagent-stoichiometry/a/limiting-reagents-and-percent-yield
- ↑ http://www.chemteam.info/Equations/Balance-Equation.html
- ↑ http://www.chemteam.info/Equations/Balance-Equation.html
- ↑ https://www.khanacademy.org/science/ap-chemistry-beta/x2eef969c74e0d802:chemical-reactions/x2eef969c74e0d802:stoichiometry/a/limiting-reagents-and-percent-yield
- ↑ https://www.khanacademy.org/science/ap-chemistry-beta/x2eef969c74e0d802:chemical-reactions/x2eef969c74e0d802:stoichiometry/a/limiting-reagents-and-percent-yield
- ↑ https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Map%3A_Introductory_Chemistry_(Tro)/08%3A_Quantities_in_Chemical_Reactions/8.06%3A_Limiting_Reactant_and_Theoretical_Yield
About This Article
To calculate theoretical yield, start by finding the limiting reactant in the equation, which is the reactant that gets used up first when the chemical reaction takes place. Then, write down the number of moles in the limiting reactant. Next, divide the number of molecules of your desired product by the number of molecules of your limiting reactant to find the ratio of molecules between them. Then, multiply the ratio by the limiting reactant's quantity in moles. Finally, convert your answer to grams. To learn how to determine the limiting reactant in the equation, continue reading the article!