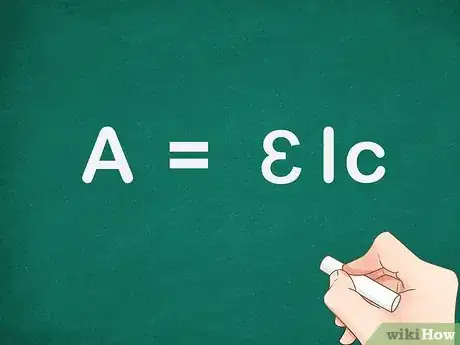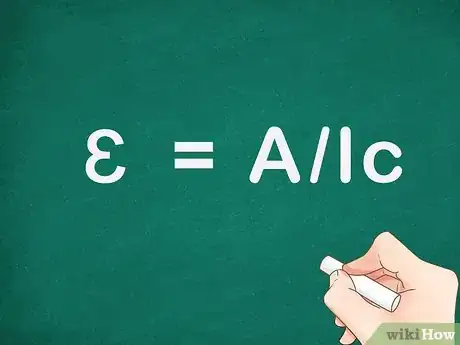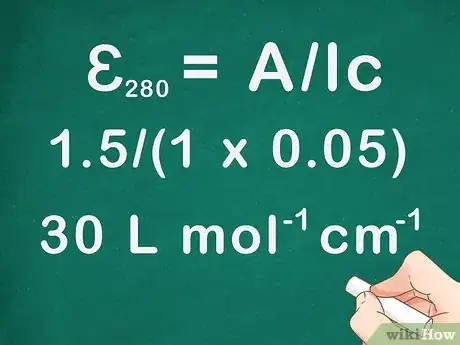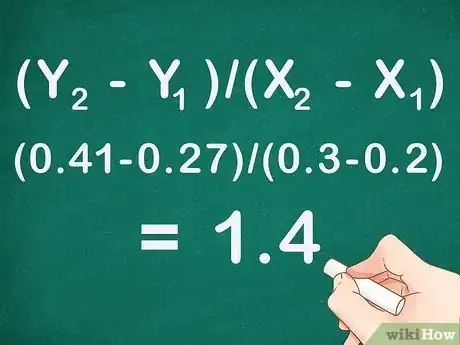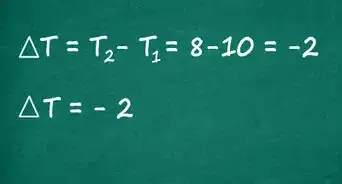This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
There are 8 references cited in this article, which can be found at the bottom of the page.
wikiHow marks an article as reader-approved once it receives enough positive feedback. This article received 23 testimonials and 87% of readers who voted found it helpful, earning it our reader-approved status.
This article has been viewed 773,912 times.
Molar absorptivity, also known as the molar extinction coefficient, is a measure of how well a chemical species absorbs a given wavelength of light. It allows you to make comparisons about the probability of electrons transition between levels for different compounds without taking into account differences in concentration or solution length during measurements.[1] It is commonly used in chemistry and should not be confused with the extinction coefficient, which is used more often in physics. The standard units for molar absorptivity are liters per mole centimeter (L mol-1 cm-1).[2]
Steps
Calculating Molar Absorptivity with the Equation
-
1Understand the Beer-Lambert law for absorbance, A = ɛ x l x c. The standard equation for absorbance is A = ɛ x l x c, where A is the amount of light absorbed by the sample for a given wavelength, ɛ is the molar absorptivity, l is the distance that the light travels through the solution, and c is the concentration of the absorbing species per unit volume.[3]
- Absorbance can also be calculated using the ratio between the intensity of a reference sample and the unknown sample. It is given by the equation A = log10(Io/I).[4]
- Intensity is obtained using a spectrophotometer.
- The absorbance of a solution will change based on the wavelength that is passed through the solution. Some wavelengths will be absorbed more than others depending upon the makeup of the solution. Remember to state which wavelength is being used for your calculation.[5]
-
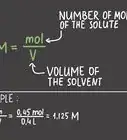
2Rearrange the Beer-Lambert equation to solve for molar absorptivity. Using algebra we can divide absorbance by the length and the concentration to get molar absorptivity on one side of the equation: ɛ = A/lc.[6] We can now use this basic equation to calculate molar absorptivity for a given wavelength.
- Absorbance between readings can vary due to the concentration of the solution and the shape of the container used to measure intensity. Molar absorptivity compensates for these variations.[7]
Advertisement -
3Obtain values for the variables in the equation using spectrophotometry. A spectrophotometer is a piece of equipment that passes a specific wavelength of light through a substance and detects the amount of light that comes out. Some of the light will be absorbed by the solution and the remaining light that passes through can be used to calculate the absorbance of that solution.
- Prepare a solution of known concentration, c, for analysis. Units for concentration are molar or moles/liter.[8]
- To find l, measure the length of the cuvette, the piece that holds the liquid samples in the spectrophotometer. Units for path length are measured in centimeters.
- Using a spectrophotometer, obtain a measurement for absorbance, A, at a given wavelength. The unit for wavelength is meters, but most wavelengths are so small, they are actually measured in nanometers (nm).[9] Absorbance has no units.
-
4Plug in the values for the variables and solve the equation for molar absorptivity. Using the values you obtained for A, c, and l, plug them into the equation ɛ = A/lc. Multiply l by c and then divide A by the product to solve for molar absorptivity.
- For example: Using a cuvette with a length of 1 cm, you measured the absorbance of a solution with a concentration of 0.05 mol/L. The absorbance at a wavelength of 280 nm was 1.5. What is the molar absorptivity of this solution?
- ɛ280 = A/lc = 1.5/(1 x 0.05) = 30 L mol-1 cm-1
- For example: Using a cuvette with a length of 1 cm, you measured the absorbance of a solution with a concentration of 0.05 mol/L. The absorbance at a wavelength of 280 nm was 1.5. What is the molar absorptivity of this solution?
Calculating Molar Absorptivity Using a Line-of-Best-Fit
-
1Measure the intensity of transmitted light through varying concentrations of solution. Make up three to four concentrations of one solution. Using a spectrophotometer, measure the absorbance of one concentration of solution at a given wavelength. Start with the lowest concentration of solution and move to the highest. The order isn’t important, but keep track of which absorbance goes with which calculation.
-
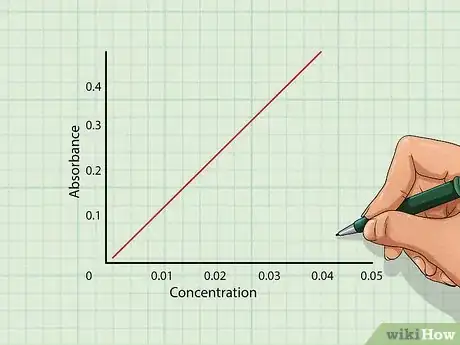
2Plot the concentration versus absorbance on a graph. Using the values obtained from the spectrophotometer, plot each point on a line graph. For each individual value, plot the concentration on the X-axis and absorbance on the Y-axis.[10]
- Draw a line between each of the points. If the measurements are correct, the points should form a straight line indicating absorbance and concentration are proportional to Beer’s Law.[11]
-
3Determine the slope of the line-of-best-fit through the data points. To calculate the slope of the line you take rise divided by run. Using two of your data points, subtract the X- and Y-values from each other, then divide Y/X.[12]
- The equation for the slope of a line is (Y2 - Y1)/(X2 - X1). The point higher on the line is given the subscript 2, while the lower point is given the subscript 1.
- For example: The absorbance at a .2 molar concentration is 0.27 and at 0.3 molar is 0.41. The absorbance values are Y-values, while concentrations are X-values. Using the equation for a line (Y2 - Y1)/(X2 - X1) = (0.41-0.27)/(0.3-0.2) = 0.14/0.1 = 1.4 is the slope of the line.
-
4Divide the slope of the line by the path length (depth of the cuvette) to calculate molar absorptivity. The final step to calculating molar absorptivity with data points is to divide by the path length. The path length is the depth of the cuvette used in the spectrophotometer.[13]
- Continuing our example: If 1.4 is the slope of the line and the path length is 0.5 cm, then the molar absorptivity is 1.4/0.5 = 2.8 L mol-1 cm-1.
Community Q&A
-
QuestionWhat is Beer Lambert's law?
 Mohsin257Community AnswerIn simple words it states that light absorbed by the sample is directly proportional to the path length (l or x) and concentration.
Mohsin257Community AnswerIn simple words it states that light absorbed by the sample is directly proportional to the path length (l or x) and concentration. -
QuestionIs the molar absorptivity constant, or does it change as the length of the cuvette changes?
 Community AnswerIt is constant. Units of molar absorptivity constant is in M^-1 cm^-1, which is essentially how much is absorbed per unit length. As the length of cuvette increases, more is absorbed as a whole, but the constant is independent of length of cuvette!
Community AnswerIt is constant. Units of molar absorptivity constant is in M^-1 cm^-1, which is essentially how much is absorbed per unit length. As the length of cuvette increases, more is absorbed as a whole, but the constant is independent of length of cuvette! -
QuestionHow do I know the path length?
 Mohsin257Community AnswerIt is known by sample compartment. Path-length is the area of the cell/sample compartment. It is mostly 1cm and depends on that compartment may be .5cm etc.
Mohsin257Community AnswerIt is known by sample compartment. Path-length is the area of the cell/sample compartment. It is mostly 1cm and depends on that compartment may be .5cm etc.
References
- ↑ http://www.chemguide.co.uk/analysis/uvvisible/beerlambert.html
- ↑ https://opg.optica.org/as/abstract.cfm?uri=as-7-1-12
- ↑ http://www.chemguide.co.uk/analysis/uvvisible/beerlambert.html
- ↑ http://www.chemguide.co.uk/analysis/uvvisible/beerlambert.html
- ↑ https://www.omnicalculator.com/chemistry/beer-lambert-law
- ↑ https://sciencing.com/calculate-molar-absorptivity-5161812.html
- ↑ http://www.chemguide.co.uk/analysis/uvvisible/beerlambert.html
- ↑ http://teaching.shu.ac.uk/hwb/chemistry/tutorials/molspec/beers1.htm
- ↑ http://www.bbc.co.uk/bitesize/standard/physics/telecommunications/communication_using_waves/revision/5/
About This Article
To calculate molar absorptivity, make sure you first understand the Beer-Lambert law for absorbance. Then, rearrange the Beer-Lambert equation into an algebraic equation so you can solve for molar absorptivity. You can obtain the values for the variables in the algebraic equation by using spectrophotometry. Once you have the values, plug them in for the variables in your equation. Once those values are plugged in, solve the algebraic equation as you normally would. The answer you get is the molar absorptivity. If you want to learn how to calculate molar absorptivity with the line-of-best-fit, keep reading the article!