This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
There are 8 references cited in this article, which can be found at the bottom of the page.
This article has been viewed 234,918 times.
Bond energy is an important concept in chemistry that defines the amount of energy needed to break a bond between a covalently bound gas.[1] This type of bond energy does not apply to ionic bonds. When 2 atoms bind together to form a new molecule, it is possible to determine how strong the bond between atoms is by measuring the amount of energy needed to break that bond. Remember, a single atom does not have a bond energy; it is the bond between 2 atoms that has energy. To calculate the bond energy of a reaction, simply determine the total number of bonds broken and then subtract the total number of bonds formed.
Steps
Determining the Broken and Formed Bonds
-
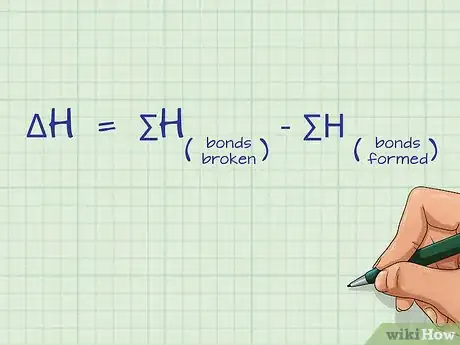
1Define the equation for calculating bond energy. Bond energy is defined by the sum of all of the bonds broken minus the sum of all of the bonds formed: ΔH = ∑H(bonds broken) - ∑H(bonds formed). ΔH is the change in bond energy, also referred to as the bond enthalpy and ∑H is the sum of the bond energies for each side of the equation.[2]
- This equation is a form of Hess’s Law.
- The unit for bond energy is kilojoules per mol or kJ/mol.[3]
-
2Draw the chemical equation showing all of the bonds between molecules. When given a reaction equation simply written with chemical symbols and numbers, it is helpful to draw this equation out, illustrating all of the bonds formed between the various elements and molecules. This visual representation will allow you to easily count all of the bonds that break and form on the reactant and product sides of the equation.
- Remember, the left side of the equation is all of the reactants and the right side is all of the products.
- Single, double, and triple bonds have different bond energies, so be sure to draw your diagram with the correct bonds between elements.[4]
- For example, if you were to draw out the following equation for a reaction between 2 hydrogen and 2 bromine: H2(g) + Br2(g) ---> 2 HBr(g), you would get: H-H + Br-Br ---> 2 H-Br. The hyphens represent single bonds between the elements in the reactants and the products.
Advertisement -
3Know the rules for counting broken and formed bonds. In most cases, the bond energies you will be using for these calculations will be averages.[5] The same bond can have a slightly different bond energy based on the molecule it is formed in; therefore, average bond energies are generally used.[6]
- A single, double, and triple bond are all treated as 1 break. They all have different bond energies, but count as only a single break.
- The same is true for the formation of a single, double, or triple bond. It will be counted as single formation.
- For our example, all of the bonds are single bonds.
-
4Identify the bonds broken on the left side of the equation. The left side contains the reactants. These will represent all of the broken bonds in the equation. This is an endothermic process that requires the absorption of energy to break the bonds.[7]
- For our example, the left side has 1 H-H bond and 1 Br-Br bond.
-
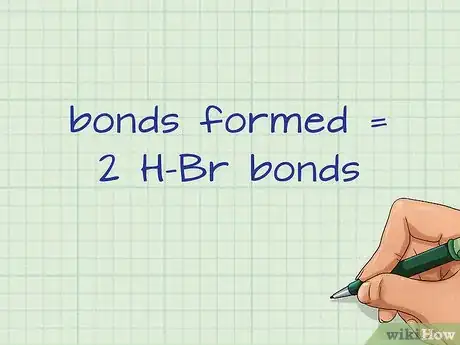
5Count the bonds formed on the right side of the equation. The right side contains all of the products. These are all of the bonds that will be formed. This is an exothermic process that releases energy, usually in the form of heat.[8]
- For our example, the right side has 2 H-Br bonds.
Calculating the Bond Energy
-
1Look up the bond energies of the bonds in question. There are many tables that have information on the average bond energies for a specific bond. These tables can be found online or in a chemistry book. It is important to note that these bond energies are always for molecules in a gaseous state.[9]
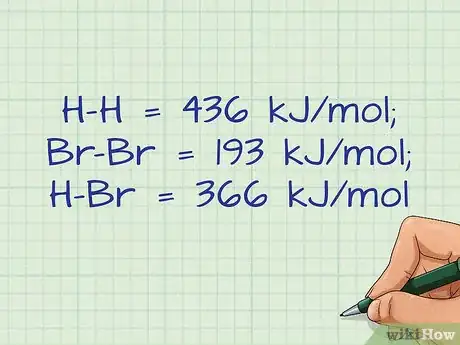
- For our example, you need to find the bond energy for an H-H bond, a Br-Br bond, and an H-Br bond.
- H-H = 436 kJ/mol; Br-Br = 193 kJ/mol; H-Br = 366 kJ/mol.
- To calculate bond energy for molecules in a liquid state, you need to also look up the enthalpy change of vaporization for the liquid molecule. This is the amount of energy needed to convert the liquid into a gas.[10]
This number is added to the total bond energy.
- For example: If you were given liquid water, you would need to add the enthalpy change of vaporization of water (+41 kJ) to the equation.[11]
-
2Multiply the bond energies by the number of bonds broken. In some equations, you may have the same bond broken multiple times.[12] For example, if 4 atoms of hydrogen are in the molecule, then the bond energy of hydrogen must be counted 4 times, or multiplied by 4.
- In our example, there is only 1 bond of each molecule, so the bond energies are simply multiplied by 1.
- H-H = 436 x 1 = 436 kJ/mol
- Br-Br = 193 x 1 = 193 kJ/mol
-
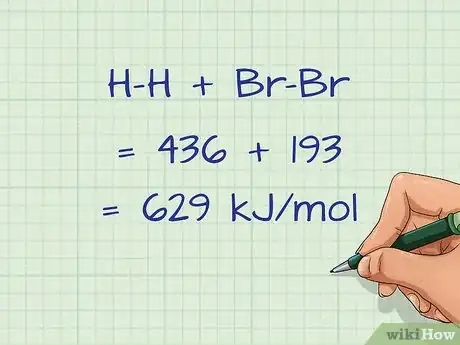
3Add up all of the bond energies of the broken bonds. Once you have multiplied the bond energies by the number of the individual bonds, you need to then sum all of the bonds on the reactant side.[13]
- For our example, the sum of the bonds broken is H-H + Br-Br = 436 + 193 = 629 kJ/mol.
-
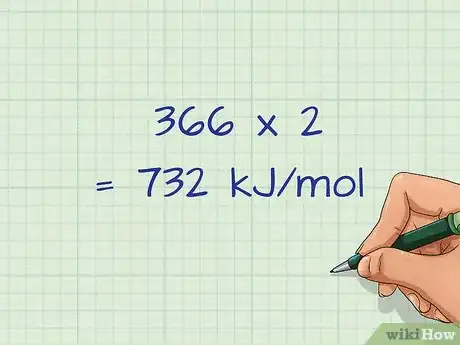
4Multiply the bond energies by the number of bonds formed. Just as you did for the bonds broken on the reactant side, you will multiply the number of bonds formed by its respective bond energy.[14] If you have 4 hydrogen bonds formed, you would need to multiply that bond energy by 4.
- For our example we have 2 H-Br bonds formed, so the bond energy of H-Br (366 kJ/mol) will be multiplied by 2: 366 x 2 = 732 kJ/mol.
-
5Add up all of the formed bond energies. Again, like you did with the bonds broken, you will add up all of the bonds formed on the product side.[15] Sometimes you will only have 1 product formed and can skip this step.
- In our example, there is only 1 product formed, so the energy of the bonds formed is simply the energy of the 2 H-Br bonds or 732 kJ/mol.
-
6Subtract the formed bonds from the broken bonds. Once you have summed all of the bond energies for both sides, simply subtract the formed bonds from the broken bonds. Remember the equation: ΔH = ∑H(bonds broken) - ∑H(bonds formed). Plug in the calculated values and subtract.[16]
- For our example: ΔH = ∑H(bonds broken) - ∑H(bonds formed) = 629 kJ/mol - 732 kJ/mol = -103 kJ/mol.
-
7Determine whether the entire reaction was endothermic or exothermic. The final step to calculating bond energy is to determine whether the reaction releases energy or consumes energy. An endothermic (one that consumes energy) will have a final bond energy that is positive, while an exothermic reaction (one that releases energy) will have a negative bond energy.[17]
- In our example, the final bond energy is negative, therefore, the reaction is exothermic.
Community Q&A
-
QuestionWhat is c-h, c-cl bond energy?
 Community Answerc-cl 339, c-h 413.
Community Answerc-cl 339, c-h 413. -
QuestionHow do I calculate energy released?
 CaeiiaTop AnswererEnergy released (exothermic reaction, when bonds are forming) is just the enthalpy of bond formation. So it is just ∑H(bonds formed).
CaeiiaTop AnswererEnergy released (exothermic reaction, when bonds are forming) is just the enthalpy of bond formation. So it is just ∑H(bonds formed). -
QuestionWhat is the bond energy of a water molecule?
 CaeiiaTop AnswererThere are two O-H bonds in water, each having about 463 kJ. 463(2) = about 1852 kJ.
CaeiiaTop AnswererThere are two O-H bonds in water, each having about 463 kJ. 463(2) = about 1852 kJ.
References
- ↑ https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/Fundamentals_of_Chemical_Bonding/Bond_Energies
- ↑ https://www.bbc.co.uk/bitesize/guides/zdy8jty/revision/3
- ↑ https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/Fundamentals_of_Chemical_Bonding/Bond_Energies
- ↑ https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/Fundamentals_of_Chemical_Bonding/Bond_Energies
- ↑ http://www.chemguide.co.uk/physical/energetics/bondenthalpies.html
- ↑ https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/Fundamentals_of_Chemical_Bonding/Bond_Energies
- ↑ https://www.khanacademy.org/science/chemistry/thermodynamics-chemistry/enthalpy-chemistry-sal/a/bond-enthalpy-and-enthalpy-of-reaction
- ↑ https://www.khanacademy.org/science/chemistry/thermodynamics-chemistry/enthalpy-chemistry-sal/a/bond-enthalpy-and-enthalpy-of-reaction
- ↑ http://www.chemguide.co.uk/physical/energetics/bondenthalpies.html
- ↑ http://www.chemguide.co.uk/physical/energetics/bondenthalpies.html
- ↑ http://www.chemguide.co.uk/physical/energetics/bondenthalpies.html
- ↑ http://www.chemteam.info/Thermochem/HessLawIntro3.html
- ↑ http://www.chemteam.info/Thermochem/HessLawIntro3.html
- ↑ https://www.bbc.co.uk/bitesize/guides/zdy8jty/revision/3
- ↑ http://www.chemteam.info/Thermochem/HessLawIntro3.html
- ↑ https://www.bbc.co.uk/bitesize/guides/zdy8jty/revision/3
- ↑ https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Equilibria/Le_Chateliers_Principle/Effect_Of_Temperature_On_Equilibrium_Composition/Exothermic_Versus_Endothermic_And_K
About This Article
To calculate bond energy, start by looking up the bond energies you’re working with either online or in a chemistry book. Then, multiply those bond energies by the number of individual bonds being broken, then add up all the energies on the reactant side of the equation. Next, repeat the process on the other side, multiplying the bond energies by the number of bonds formed and adding them up. Finally, subtract the formed bonds from the broken bonds to get the bond energy. If your answer is positive, the bond is endothermic, and if your answer is negative, the bond is exothermic. For tips on calculating the bond energy for liquid molecules, read on!
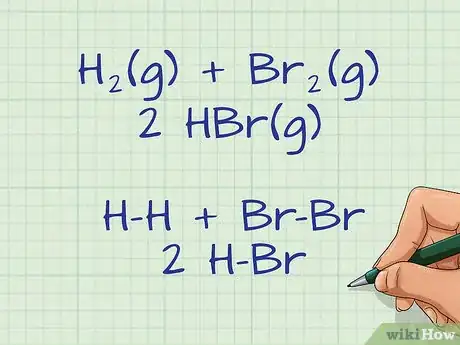


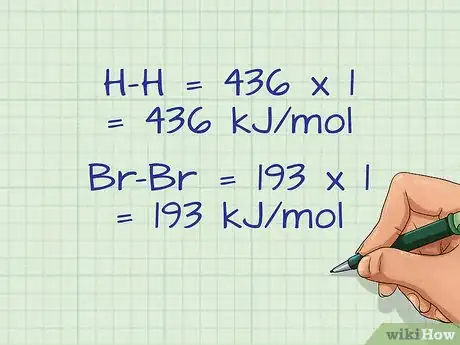



-Step-18.webp)















-Step-18.webp)