This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
There are 11 references cited in this article, which can be found at the bottom of the page.
This article has been viewed 696,255 times.
In chemistry, electronegativity is a measure of how strongly an atom attracts the electrons in a bond.[1] An atom with high electronegativity attracts electrons strongly, while an atom with low electronegativity attracts them weakly. Electronegativity values are used to predict how different atoms will behave when bonded to each other, making this an important skill in basic chemistry.
Steps
Electronegativity Basics
-
1Understand that chemical bonds occur when atoms share electrons. To understand electronegativity, it's important first to understand what a "bond" is. Any two atoms in a molecule that are "connected" to each other on a molecular diagram are said to have a bond between them.[2] This means that they share a set of two electrons with each atom contributing one electron to the bond.
- The exact reasons for why atoms share electrons and bonds are a little beyond the scope of this article. If you want to learn more, try this article on the bond basics or WikiHow's own How to Study the Nature of the Chemical Bond (Chemistry).
-
2Understand how electronegativity affects the electrons in the bond. When two atoms share a set of two electrons in a bond, they don't always share them equally. When one atom has a higher electronegativity than the atom it's bonded to, it pulls the two electrons in the bond closer to itself. An atom with very high electronegativity may pull the electrons all the way to its side of the bond, barely sharing them at all with the other atom.[3]
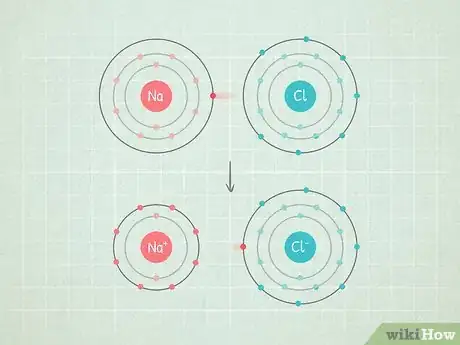
- For example, in the molecule NaCl (sodium chloride), the chlorine atom has a fairly high electronegativity and the sodium has a fairly low one. Thus, the electrons will get pulled towards the chloride and away from the sodium.
Advertisement -
3Use an electronegativity table as a reference. An electronegativity table of the elements has the elements arranged exactly like in a periodic table, except that each atom is labeled with its electronegativity. These can be found in a variety of chemical textbooks and technical articles as well as online.
-
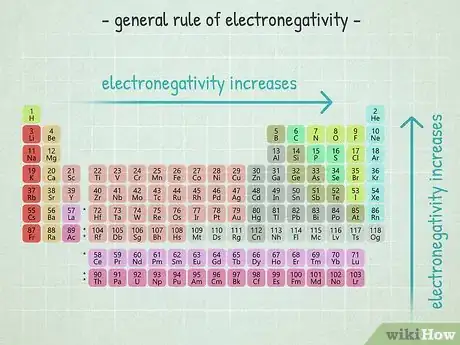
4Remember electronegativity trends for easy estimations. If you don't have an electronegativity table handy, you can still estimate the strength of an atom's electronegativity compared to the strength of another element's atom based on where it is located on a normal periodic table.[5] Although you will not be able to calculate a number value, you can evaluate the difference between the electronegativities of 2 different elements. As a general rule:
- An atom's electronegativity gets higher as you move to the right in the periodic table.
- An atom's electronegativity gets higher as you move up in the periodic table.
- Thus, the atoms in the top right have the highest electronegativities and the atoms in the bottom left have the lowest ones.
- For example, in the NaCl example from above, you can tell that chlorine has a higher electronegativity than sodium because it's almost all the way in the top right. On the other hand, sodium is far to the left, making it one of the lower-ranking atoms.
Finding Bonds With Electronegativity
-
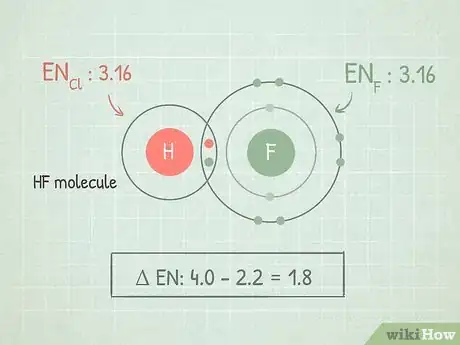
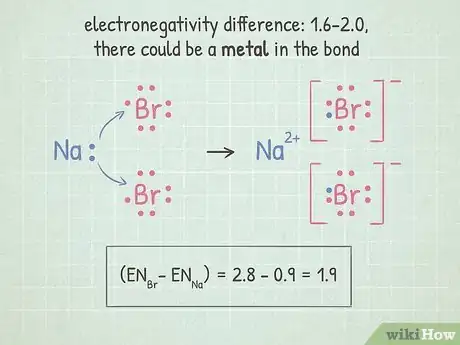
1Find the electronegativity difference between the two atoms. When two atoms are bonded together, the difference between their electronegativities can tell you about the qualities of their bond. Subtract the smaller electronegativity from the larger one to find the difference.
- For example, if we're looking at the molecule HF, we would subtract the electronegativity of hydrogen (2.1) from fluorine (4.0). 4.0 - 2.1 = 1.9
-
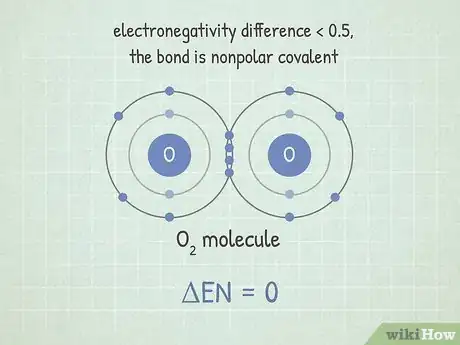
2If the difference is below about 0.5, the bond is nonpolar covalent. Here, the electrons are shared almost equally. These bonds don't form molecules that have large charge differences on either end. Nonpolar bonds tend to be very difficult to break.[6] This is because the atoms are sharing electrons, making their bond stable. It requires a lot of energy to break this bond.[7]
- For example, the molecule O2 has this type of bond. Since the two oxygens have the same electronegativity, the difference between them is 0.
-
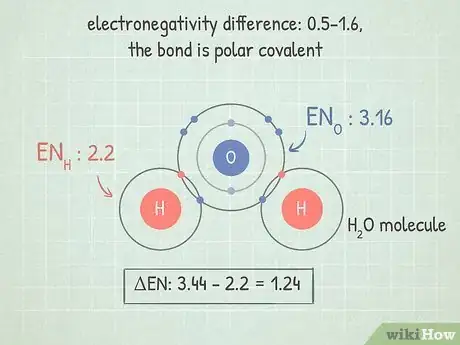
3If the difference is between 0.5-1.6, the bond is polar covalent. These bonds have more electrons at one end than the other. This makes the molecule a little more negative at the end with the electrons and a little more positive at the end without them. The charge imbalance in these bonds can allow the molecule to participate in certain special reactions, such as joining with another atom or molecule or pulling a molecule apart. This is because it's still reactive.[8]
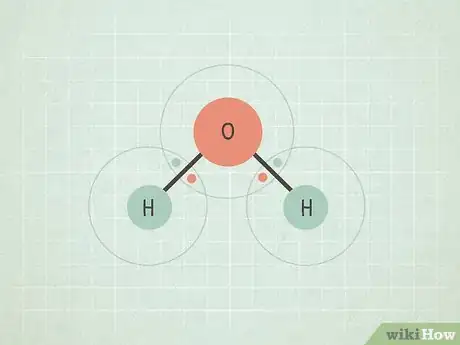
- A good example of this is the molecule H2O (water). The O is more electronegative than the two Hs, so it holds the electrons more tightly and makes the entire molecule partially negative at the O end and partially positive at the H ends.
-
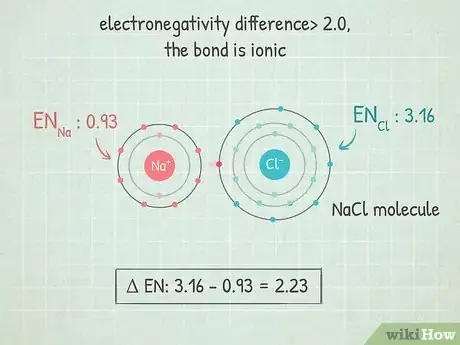
4If the difference is over 2.0, the bond is ionic. In these bonds, the electrons are completely at one end of the bond. The more electronegative atom gains a negative charge and the less electronegative atom gains a positive charge. These sorts of bonds allow their atoms to react well with other atoms and even be pulled apart by polar molecules.
- An example of this is NaCl (sodium chloride or salt). The chlorine is so electronegative that it pulls both electrons in the bond all the way towards itself, leaving sodium with a positive charge.
- NaCl can be broken apart by a polar molecule, such as H2O (water). In a water molecule, the hydrogen side of the molecule is positive, while the oxygen side is negative. When you mix the salt into the water, the water molecules break down the salt molecules, dissolving the salt.[9]
-
5If the difference is between 1.6-2.0, check for a metal. If there is a metal in the bond, the bond is ionic. If there are only non-metals, the bond is polar covalent.
Find the Mulliken Electronegativity
-
1Find the first ionization energy of your atom. Mulliken electronegativity is a slightly different way of measuring electronegativity than is used in the Pauling table above. To find Mulliken electronegativity for a certain atom, find that atom's first ionization energy. This is the energy required to make the atom discharge a single electron.
- This is something you'll probably have to look up in chemistry reference materials. This site has a good table you may want to use (scroll down to find it).[11]
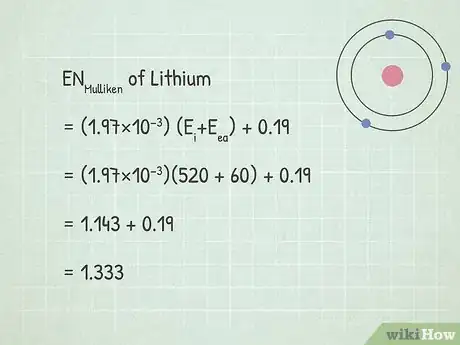
- As an example, let's say that we're trying to find the electronegativity of lithium (Li). In the table on the site above, we can see that its first ionization energy is 520 kJ/mol.
-
2Find the electron affinity of the atom. This is a measure of the energy gained when an electron is added to an atom to form a negative ion. Again, this is something you'll need to look up in reference material. This site has resources you may want to browse.[12]
- The electron affinity of lithium is 60 kJ mol-1.
-
3Solve the Mulliken electronegativity equation. When you're using kJ/mol as units for your energies, the equation for Mulliken electronegativity is ENMulliken = (1.97×10−3)(Ei+Eea) + 0.19. Plug your values into the equation and solve for ENMulliken.[13]
- In our example, we would solve like this:
- ENMulliken = (1.97×10−3)(Ei+Eea) + 0.19
- ENMulliken = (1.97×10−3)(520 + 60) + 0.19
- ENMulliken = 1.143 + 0.19 = 1.333
- In our example, we would solve like this:
Community Q&A
-
QuestionWhen dipole movement is zero, will the compound be more or less stable?
 Community AnswerLess stable; dipole movement gives the compounds more stability thanks to intramolecular resonance.
Community AnswerLess stable; dipole movement gives the compounds more stability thanks to intramolecular resonance. -
QuestionWhat is the electronegativity of n-h?
 Community AnswerThe electronegativity of n-h is 3.194.
Community AnswerThe electronegativity of n-h is 3.194. -
QuestionWhat is electron affinity?
 Skanda PrasadCommunity AnswerElectron affinity is the amount of energy released or absorbed when an atom in gaseous state accepts an electron to form an anion (not necessarily an anion but yes it should accept an electron). If energy is released it is exothermic, if energy is absorbed, it is endothermic.
Skanda PrasadCommunity AnswerElectron affinity is the amount of energy released or absorbed when an atom in gaseous state accepts an electron to form an anion (not necessarily an anion but yes it should accept an electron). If energy is released it is exothermic, if energy is absorbed, it is endothermic.
References
- ↑ http://www.chemguide.co.uk/atoms/bonding/electroneg.html
- ↑ https://chemistrytalk.org/what-is-a-chemical-bond/
- ↑ https://chemistrytalk.org/what-is-a-chemical-bond/
- ↑ http://www.tutor-homework.com/Chemistry_Help/electronegativity_table/electronegativity.html
- ↑ https://chemistrytalk.org/electronegativity-chart-trends/
- ↑ http://study.com/academy/lesson/polar-and-nonpolar-covalent-bonds-definitions-and-examples.html
- ↑ http://www.rsc.org/Education/Teachers/Resources/cfb/water.htm
- ↑ http://study.com/academy/lesson/polar-and-nonpolar-covalent-bonds-definitions-and-examples.html
- ↑ https://water.usgs.gov/edu/solvent.html
- ↑ http://www.tutor-homework.com/Chemistry_Help/electronegativity_table/electronegativity.html
- ↑ http://chemwiki.ucdavis.edu/Physical_Chemistry/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Ionization_Energy
- ↑ http://chemwiki.ucdavis.edu/Physical_Chemistry/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Electron_Affinity
- ↑ https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Electronegativity/Mulliken_Electronegativity
- http://dl.clackamas.edu/ch104-07/electron.htm
About This Article
Electronegativity is a measure of how strongly an atom attracts electrons in a bond with another atom. To understand the nature of a bond between two atoms, look up the electronegativity of each atom on an electronegativity table or a periodic table that lists electronegativity. Keep in mind that electronegativity gets higher as you move up and to the right on the chart. Once you’ve found the electronegativity of each atom, subtract the smaller electronegativity from the larger one to find the difference. For instance, in a bonded pair of hydrogen and fluorine atoms, hydrogen has an electronegativity of 2.1, while fluorine has an electronegativity of 4.0. The difference between them is 1.9. In molecules where the difference is less than 0.5, the bond is nonpolar covalent. This is a strong type of bond that takes a lot of energy to break. If the difference is between 0.5 and 1.6, the bond is polar covalent. These bonds are imbalanced and make the resulting molecule more reactive than one with a nonpolar covalent bond. If the difference is greater than 2.0, the bond is ionic, which means that one atom has a positive charge and the other has a negative one. These types of bonds are easy to break, meaning the molecule is very reactive. For electronegativity differences between 1.6 and 2.0, the bond could be either polar covalent or ionic. If there’s a metal in the molecule, the bond is ionic. If both atoms are non-metals, the bond is polar covalent instead. To learn more about calculating electronegativity by using the Mulliken equation, scroll down!