This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
There are 7 references cited in this article, which can be found at the bottom of the page.
This article has been viewed 186,564 times.
A great way to write configuration is to try making a song out of it. Writing out an electron configuration for an element is a great way to look at the distribution of electrons in an atom. Depending on the element, it may be very long. Because of this, scientists have developed a shorthand notation that involves using a noble gas to represent electrons that are not valence electrons. This simplifies the electron configuration and makes it easier to understand the chemistry of the element.[1]
Steps
Writing the Normal Electron Configuration of an Element
-
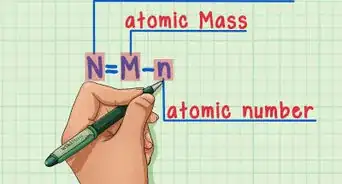
1Identify the number of electrons present in the element. The atomic number of an element tells you the number of protons it has. Because elements in their neutral state have the same number of protons and electrons, you can also use the atomic number as the number of electrons the element has. The atomic number, which can be found on the periodic table, is the number written directly above the symbol for the element.
- For example, the symbol for sodium is Na. The atomic number for Na is 11.
-
2Know about electron shells and energy levels. The first electron shell has only the s energy level, the second electron shell has both an s and p energy level. The third electron shell has an s, p, and d energy level. The fourth electron shell has an s, p, d, and f energy level. There are more than four electron shells, but for a standard chemistry course you will generally only use the first four.[2]
- Each s energy level can hold a maximum of 2 electrons.
- Each p energy level can hold a maximum of 6 electrons.
- Each d energy level can hold a maximum of 10 electrons.
- Each f energy level can hold a maximum of 14 electrons.
Advertisement -
3Learn the rules for electron filling. According to the Aufbau principle, you must add electrons to the lowest energy levels before an electron can be added to a higher energy level. Each energy level may have multiple suborbitals, but each suborbital can hold a maximum of two electrons at any given time. The s energy level has one suborbital, p has 3 suborbitals, d has 5 suborbitals, and f has 7 suborbitals.[3]
- The d energy level has a slightly higher energy than the s energy level of the lower electron shell, so the higher s energy level will fill before the lower d energy level. For writing an electron configuration this means it will look like this: 1s22s22p63s23p64s23d10.
-
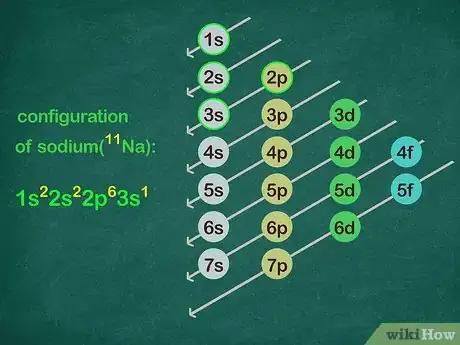
4Use the diagonal configuration chart to write electron configurations. The easiest way to remember how electrons fill is to use the configuration chart. This is where you write out each shell and the energy levels within it. Draw diagonal lines from the top right through to the bottom left of each line. The configuration chart looks like this:[4]
- 1s
2s 2p
3s 3p 3d
4s 4p 4d 4f
5s 5p 5d 5f
6s 6p 6d
7s 7p - For example: The electron configuration of sodium (11 electrons) is 1s22s22p63s1.
- 1s
-
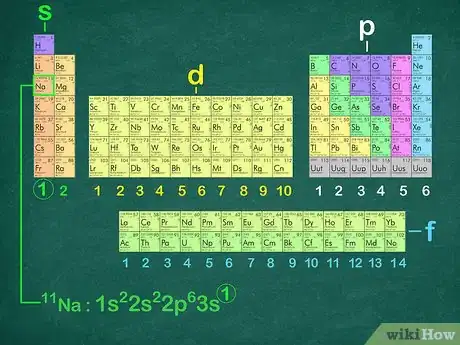
5Recognize what the last orbital of each configuration will be. By looking at the periodic table, you can determine what the last subshell and energy level of the electron configuration will be. First determine which block the element falls in (s, p, d, or f). Then count which row the element is in. Finally, count which column the element is in.[5]
- For example, sodium is in the s block, so the last orbital of its electron configuration will be s. It is in the third row and the first column, therefore the last orbital is 3s1. This is a good way to double-check your final answer.
- The rule is a little bit different for the d orbital. The first row of d-block elements starts in the fourth row, but you must subtract 1 from the row number because the s levels are lower energy than the d levels. For example, vanadium ends with 3d3.[6]
- Another way to double check your work is to add all of the superscripts together. They should equal the number of electrons in the element. If you have too few or too many electrons, you will need to look over your work and try again.
Writing the Noble Gas Electron Configuration
-
1Understand the noble gas electron configuration. The noble gas electron configuration is a type of shortcut to writing out the full electron configuration of an element. The noble gas shorthand is used to summarize the electron configuration of an element while providing the most relevant information about the valence electrons of that element.[7]
- The noble gas is substituted to represent all of the electrons that aren’t valence electrons.
- The noble gases are helium, neon, argon, krypton, xenon, and radon and are found in the last column of the periodic table.
-
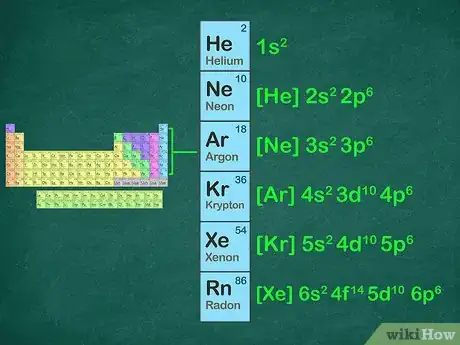
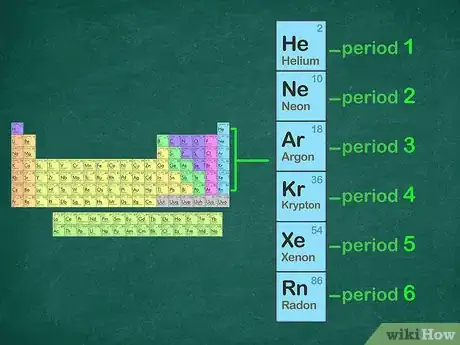
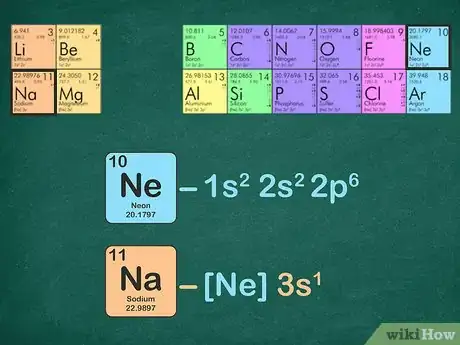
2Identify the noble gas in the period before your element. The period of an element is the horizontal row that the element is located in. If the element is in the fourth row of the periodic table, it is in period four. The noble gas you will use will be located in period three. Below is a list of the noble gases and their periods:[8]
- 1: Helium
- 2: Neon
- 3: Argon
- 4: Krypton
- 5: Xenon
- 6: Radon
- For example, sodium is in period three. We will use neon for the noble gas configuration because it is in period 2.
-
3Substitute the noble gas for the same number of electrons the noble gas has. There are a few ways to do this next step. You can physically write out the electron configuration for the noble gas and then replace that same configuration in your element of interest. An alternative is to remove the same number of electrons the noble gas has from the element you are writing the configuration for.[9]
- For example, sodium has 11 electrons and neon has 10 electrons.
- The full electron configuration for sodium is 1s2s222p63s1 and neon is 1s2s222p6. As you can see, sodium has a 3s1 that neon does not have, therefore, the noble gas configuration for sodium would be [Ne]3s1.
- Alternatively, you can count the superscripts of the energy levels until you get to ten. Remove these energy levels and leave what remains. When using neon to write the electron configuration for sodium, you will have one electron leftover: [Ne]3s1.
Community Q&A
-
QuestionWhat is the noble gas notation for lead?
 Community AnswerThe noble gas notation for lead is [Xe]6s2 4f14 5d10 6P2.
Community AnswerThe noble gas notation for lead is [Xe]6s2 4f14 5d10 6P2. -
QuestionWhat is the noble gas configuration for a noble gas? Would you go to the previous noble gas or simply put in brackets?
 Community AnswerYou would use the noble gas before it. The noble gas for Neon (Ne) is [He] 2s2 2p6.
Community AnswerYou would use the noble gas before it. The noble gas for Neon (Ne) is [He] 2s2 2p6. -
QuestionWhat are noble gases?
 Community AnswerAny element in column 16. They also have eight valence electrons, so they don't need to gain or lose electrons.
Community AnswerAny element in column 16. They also have eight valence electrons, so they don't need to gain or lose electrons.
Warnings
- Only in a neutral atom does the atomic number equal the number of electrons. An ion contains a different number of electrons. If the ion has a negative one charge, it will have one extra electron. A negative two charge has two extra electrons, etc.⧼thumbs_response⧽
References
- ↑ https://socratic.org/chemistry/the-electron-configuration-of-atoms/noble-gas-shorthand
- ↑ http://www.chemtube3d.com/A%20Level%20orbitals.htm
- ↑ http://www.iun.edu/~cpanhd/C101webnotes/modern-atomic-theory/aufbau-principle.html
- ↑ http://terpconnect.umd.edu/~wbreslyn/chemistry/electron-configurations/
- ↑ http://terpconnect.umd.edu/~wbreslyn/chemistry/electron-configurations/
- ↑ https://youtu.be/Nz7C08t1eSc?t=3m54s
- ↑ https://socratic.org/chemistry/the-electron-configuration-of-atoms/noble-gas-shorthand
- ↑ http://www.ck12.org/chemistry/Noble-Gas-Configuration/lesson/Noble-Gas-Configuration-CHEM/
- ↑ https://www.youtube.com/watch?v=AOxaVdA-Sv0