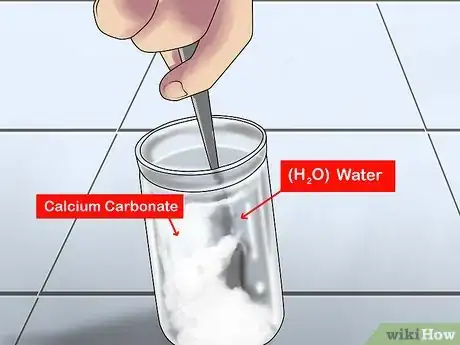This article was co-authored by Meredith Juncker, PhD. Meredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
There are 8 references cited in this article, which can be found at the bottom of the page.
wikiHow marks an article as reader-approved once it receives enough positive feedback. In this case, several readers have written to tell us that this article was helpful to them, earning it our reader-approved status.
This article has been viewed 331,151 times.
Carbon dioxide (CO2) is colorless and odorless, so you can't detect it through direct observation. You'll need to collect an air sample (or a CO2 sample), then run one of several simple tests to identify the presence of the gas. You can bubble the gas through limewater, or you can hold a lit splint into the sample to see if it is extinguished by the presence of CO2.
Steps
Preparing a Sample
-
1Collect a CO2 sample. To begin your test, you will need a sealed test tube filled with collected gas. You can collect carbon dioxide in a gas jar, a boiling tube, or another airtight container. Collection is usually performed over water in a beaker. CO2 gas is denser than air, so you can collect it using "downward delivery" or a gas syringe.
-
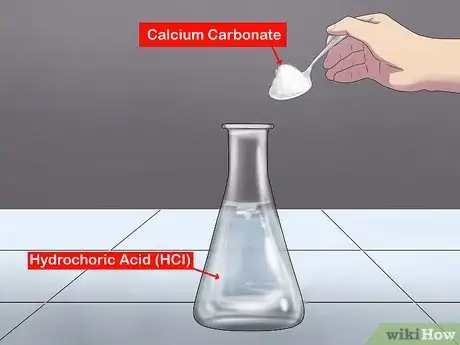
2Mix calcium carbonate with hydrochloric acid (HCl). The simplest way to collect carbon dioxide is to react calcium carbonate (or limestone chips) with hydrochloric acid. First, pour 20 mL of HCl into a conical flask. Add a spoonful of calcium carbonate (or limestone chips) to the HCL. When the reaction starts, cover the conical flask with a bung and delivery tube: you will collect the gas through the delivery tube and into an upturned test tube (which is immersed in bowl of water). If the water in the test tube is displaced, then gas is being collected.[1]
- You can continue to collect the gas for as long as the reaction occurs.
- For classroom demonstrations, you only need a small amount of hydrochloric acid, which is diluted to 1 M; a 2 M concentration works best, but should be used with extra care because it's a strong acid. The equation is: CaCO3(s) + 2HCl(aq) ==> CaCl2(aq) + H2O(l) + CO2(g).
- Be very careful when working with hydrochloric acid – wear gloves, a lab coat, and protective goggles, and do not let the acid touch your skin! It's best to only perform this reaction if you have access to a lab environment.[2]
Advertisement -
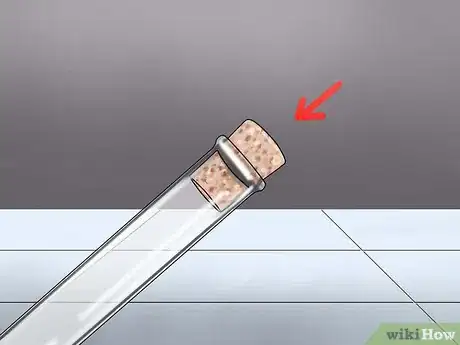
3Cover the test tube with a bung. Put the tube on a rack to keep it safe until you perform the test. The "bung" is essentially a small cork or cap that allows you to pipe the contents of the test tube elsewhere through a connected delivery tube. It's important to seal the CO2 gas into the container. If you leave it open, the gas will mix with the air, and your test will be much less effective.
Bubbling Through Limewater
-
1Bubble the gas through limewater. The most effective way to test for CO2 is to bubble the gas through "limewater", a diluted solution of calcium hydroxide (slaked lime). When you bubble carbon dioxide through the solution, it forms a solid precipitate of calcium carbonate – chalk or limestone. Calcium carbonate is insoluble in water.[3] Thus, if there is CO2 present in the sample, the limewater will turn milky, cloudy white.[4]
- Limewater is also called "white wash" or "milk of lime." If you see these terms, you'll know they mean limewater.[5]
-
2Make a limewater solution. The process is simple: dilute calcium hydroxide with water. Calcium hydroxide (Ca(OH)2) is a colorless white powder that you can buy from most chemistry suppliers. Pure limewater, once mixed, is clear and colorless, with a slight earthy smell and the bitter, alkaline taste of calcium hydroxide, as its pH is 12.3. Follow these steps to make your own limewater:
- Put 1 teaspoon (4.9 mL) of calcium hydroxide into a clean 1 gallon (3.8 L) or smaller glass jar. Limewater is a saturated solution, which means there will be some extra chemical that doesn't dissolve. A teaspoon will result in a fully saturated solution, whether you use a gallon jar or a smaller container.
- Fill the jar with distilled water. It's best to use distilled water for this experiment, as it will give you a purer solution.
- Put a lid on the jar. Shake the solution vigorously for 1-2 minutes, then let it stand for 24 hours.
- Pour the clearer solution off the top of the jar through a clean coffee filter or filter paper. Be very careful not to stir up the sediment. If necessary, repeat this filtering step until you obtain a clear limewater solution. Store in a clean jar or bottle.
-
3Bubble the gas through the limewater. Half-fill a test tube with limewater – then boil it. Use a delivery tube to pipe the contents of the CO2 sample test tube directly into the boiling limewater. You can use a flexible pipe or a (metal) straw as a delivery tube, if nothing better is around. Let the captured gas "bubble" through the liquid, and wait for the reaction to take place.
- If you don't want to boil anything, you can use a gas syringe to discharge the CO2 gas directly into the half-filled limewater test tube. Stopper the test tube, then shake vigorously for 1-2 minutes. If there is carbon dioxide in the sample, then the solution should grow cloudy.[6]
-
4Look for cloudy water. If CO2 is present, the limewater will turn milky white with calcium carbonate particulates, as the calcium carbonate solution is insoluble and will precipitate out of the solution. If the limewater is boiling, and the gas is piped directly into the limewater, then the reaction should begin immediately. If nothing happens after a minute or so, you can safely assume that there is no carbon dioxide in your sample.[7]
-
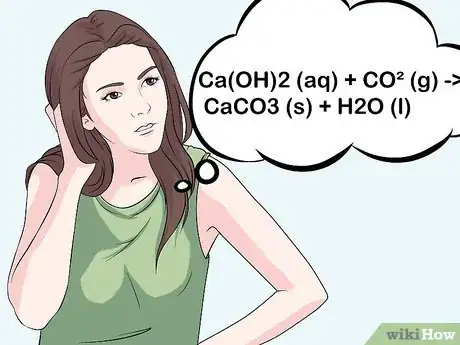
5Know the chemical reaction. Understand what exactly is taking place to indicate the presence of CO2. The equation for the test is: Ca(OH)2 (aq) + CO2 (g) ==> CaCO3 (s) + H2O (l). In non-chemistry language: liquid limewater + gas (which contains CO2) react to solid lime (the particles) and liquid water.[8]
- If you bubble the CO2 through the limewater for an extended period of time, your water will eventually turn clear. That's because of a chemical reaction! Here's why:
- CO2 + H2O ==> H2CO3 (carbon dioxide + water = carbonic acid)
- H2CO3 + CaCO3 ==> Ca(HCO3)2 (carbonic acid + calcium carbonate = calcium hydrogen carbonate)
- Calcium hydrogen carbonate is soluble in water, making your solution clear!
- If you bubble the CO2 through the limewater for an extended period of time, your water will eventually turn clear. That's because of a chemical reaction! Here's why:
Testing with a Lit Splint
-
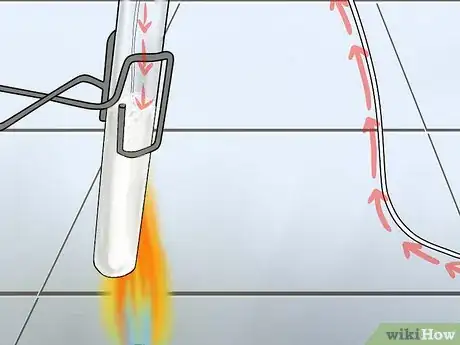
1Try using the sample to extinguish fire. Carbon dioxide extinguishes fires, in high concentrations. You'll simply need to hold a small lit flame inside a test tube that you suspect contains CO2. If the gas is present, the flame should go out immediately. Combustion (creation of a flame) is the reaction of oxygen with another substance; it is a rapid oxidation of the organic compound and a reduction of oxygen. The fire goes out because the oxygen is replaced by the CO2, which is not a combustible gas.
- Bear in mind that any oxygen-free gas will also extinguish a flame in this manner. Thus, this is an unreliable test for carbon dioxide, and it may lead you to misidentify the gas.[9]
-
2Collect the gas in an upside-down test tube. Make sure that the sample has been properly stored and stoppered before you try to test for CO2. Be reasonably certain that the test tube does not contain any flammable or explosive gases; in this case, the introduction of fire could be dangerous, or at least very frightening.
-
3Place a small flame inside the test tube. Use a splint or any long, thin strip of wood. In a pinch, a match or a lighter will do – but the further your hands are from the opening of the test tube, the safer your experiment will be. If the flame immediately goes out, there's likely a high concentration of CO2 in the test tube.
-
4Alternately, try using a gas syringe to put out a candle. Fill a syringe with carbon dioxide. Then, use a drop of molten wax to affix a short candle to the surface of a coin. Next, place the candle and coin into a wide-mouthed cup – and light the candle. Equip the syringe with the tubing, and push the syringe to transfer the CO2 to the bottom of the cup. If you push out the entire contents of the syringe within a second or two, the flame should go out.
Expert Q&A
Did you know you can get expert answers for this article?
Unlock expert answers by supporting wikiHow
-
QuestionWhy should the test for carbon dioxide be performed immediately after collection of water sample?
 Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Scientific Researcher
-
QuestionIs carbon dioxide harmful to humans?
 Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Scientific Researcher It depends on how much carbon dioxide is present. In small amounts, it's not harmful to humans. However, if a large amount of carbon dioxide is present and displaces the oxygen in the air, it can cause respiratory issues for humans. The lack of oxygen can cause a person to breath rapidly and experience rapid heartbeat. If the lack of oxygen happens for a long time, the person might have damage to the brain, heart, and other organs.
It depends on how much carbon dioxide is present. In small amounts, it's not harmful to humans. However, if a large amount of carbon dioxide is present and displaces the oxygen in the air, it can cause respiratory issues for humans. The lack of oxygen can cause a person to breath rapidly and experience rapid heartbeat. If the lack of oxygen happens for a long time, the person might have damage to the brain, heart, and other organs. -
QuestionWhat happens to the calcium hydroxide solutions when carbon dioxide bubbles through it?
 Community AnswerCalcium hydroxide, or limewater, will turn milky/cloudy when carbon dioxide is bubbled through it.
Community AnswerCalcium hydroxide, or limewater, will turn milky/cloudy when carbon dioxide is bubbled through it.
Things You'll Need
- Limewater
- Heat-able test tube
- Gas syringe
- Delivery tube
- CO2 sample (or just water)
References
- ↑ https://earlieuk.wordpress.com/2011/02/18/how-to-collect-and-test-oxygen-hydrogen-and-carbon-dioxide/
- ↑ http://www.docbrown.info/page13/ChemicalTests/GasPreparation.htm#Ex 4.
- ↑ http://chemstuff.co.uk/analytical-chemistry/tests-for-gases/
- ↑ https://www.education.com/science-fair/article/gas-sniffers/
- ↑ https://sciencestruck.com/how-to-make-lime-water
- ↑ https://www.youtube.com/watch?v=pCDAXJyrABw
- ↑ https://www.youtube.com/watch?v=5FcdMQDzvEo
- ↑ http://antoine.frostburg.edu/chem/senese/101/inorganic/faq/co2-detection.shtml
- ↑ http://chemstuff.co.uk/analytical-chemistry/tests-for-gases/
About This Article
To test for carbon dioxide, start by diluting some calcium hydroxide with water to make a limewater solution. Then, fill up a test tube halfway with the limewater and bring it to a boil. Next, use a delivery tube to pipe the contents of your sample into the boiling limewater. If the limewater turns cloudy and milky white, your sample has carbon dioxide in it. If no change occurs, there's no carbon dioxide in the sample. To learn how to collect a carbon dioxide sample, keep reading!