This article was co-authored by Cleanzen Cleaning Services. The Cleanzen Cleaning Services Team consists of Residential Cleaning Specialists. With more than six years of experience, they specialize in connecting independent cleaning professionals with those who need help cleaning their houses. All of Cleanzen’s professionals are experienced and licensed and have passed background checks.
There are 8 references cited in this article, which can be found at the bottom of the page.
wikiHow marks an article as reader-approved once it receives enough positive feedback. This article received 16 testimonials and 100% of readers who voted found it helpful, earning it our reader-approved status.
This article has been viewed 424,281 times.
Corrosion can be a huge problem for anyone who works with metals, from small projects to large-scale constructions like buildings, bridges, and aircraft. All metals will corrode eventually, but fortunately, we have a few tips you can use to help slow down this process.
Steps
Understanding Common Types of Metal Corrosion
Because so many different types of metal are in use today, builders and manufacturers need to protect against many different types of corrosion. Every metal has its own unique electrochemical properties which determine which types of corrosion (if any) the metal is vulnerable to. The table below details a selection of common metals and the types of corrosion they can undergo.
| Metal | Metal's Corrosion Vulnerability(s) | Common Preventative Techniques | Galvanic Activity* |
|---|---|---|---|
| Stainless Steel (Passive) | Uniform attack, galvanic, pitting, crevice (all esp. in saltwater)[1] | Cleaning, protective coating or sealant | Low (initial corrosion forms resistant oxide layer) |
| Iron | Uniform attack, galvanic, crevice | Cleaning, protective coating or sealant, galvanization, anti-rust sol'ns | High |
| Brass | Uniform attack, dezincification, stress | Cleaning, protective coating or sealant (usually oil or lacquer), adding tin, aluminum, or arsenic to alloy[2] | Medium |
| Aluminum | Galvanic, pitting, crevice[3] | Cleaning, protective coating or sealant, anodizing, galvanizing, cathodic protection, electrical insulation [4] | High (initial corrosion forms resistant oxide layer) |
| Copper | Galvanic, pitting, aesthetic tarnishing | Cleaning, protective coating or sealant, adding nickel to alloy (esp. for saltwater) | Low (initial corrosion forms resistant patina) |
*Note that the "Galvanic Activity" column refers to the metal's relative chemical activity as described by galvanic series tables from reference sources.[5] For the purposes of this table, the higher the metal's galvanic activity, the more quickly it will undergo galvanic corrosion when joined with a less-active metal.
-
1Prevent uniform attack corrosion by protecting the metal surface. Uniform attack corrosion (sometimes shortened to "uniform" corrosion) is a type of corrosion that occurs, appropriately, in a uniform fashion over an exposed metal surface. In this type of corrosion, the entire surface of the metal is under attack from corrosion and, thus, the corrosion proceeds at a uniform rate. For example, if an unprotected iron roof is regularly exposed to rain, the entire roof surface will come into contact with roughly the same amount of water and thus will corrode at a uniform rate. The easiest way to protect against uniform attack corrosion is usually to put a protective barrier between the metal and the corroding agents. This can be a wide variety of things - paint, an oil sealant, or an electrochemical solution like a galvanized zinc coating.
- In underground or immersion situations, cathodic protection is also a good choice.[6]
-
2Prevent galvanic corrosion by halting ion flow from one metal to another. One important form of corrosion that can occur regardless of the physical strength of the metals involved is galvanic corrosion. Galvanic corrosion occurs when two metals with differing electrode potentials are in contact with one another in the presence of an electrolyte (like saltwater) that creates an electrical conducting path between the two. When this happens, metal ions flow from the more-active metal to the less-active metal, causing the more-active metal to corrode at an accelerated rate and the less-active metal to corrode at a slower rate. In practical terms, this means that corrosion will develop on the more-active metal at the point of contact between the two metals.
- Any method of protection that prevents ion flow between the metals can potentially halt galvanic corrosion. Giving the metals a protective coating can help prevent electrolytes from the environment from creating an electrical conducting path between the two metals, while electrochemical protection processes like galvanization and anodizing also work well. It's also possible to thwart galvanic corrosion by electrically insulating the areas of the metals that come into contact with each other.
- Additionally, the use of cathodic protection or a sacrificial anode can protect important metals from galvanic corrosion. See below for more information.
Advertisement -
3Prevent pitting corrosion by protecting the metal surface, avoiding environmental chloride sources, and avoiding nicks and scratches. Pitting is a form of corrosion that takes place at the microscopic scale but can have large-scale consequences. Pitting is of great concern for metals that derive their corrosion resistance from a thin layer of passive compounds on their surface, as this form of corrosion can lead to structural failures in situations where the protective layer would normally prevent them. Pitting occurs when a small part of the metal loses its protective passive layer. When this happens, galvanic corrosion occurs at a microscopic scale, leading to the formation of a tiny hole in the metal. Within this hole, the local environment becomes highly acidic, which accelerates the process. Pitting is usually prevented by applying a protective coating to the metal surface and/or using cathodic protection.
- Exposure to an environment high in chlorides (like, for example, salt water) is known to accelerate the pitting process.
-
4Prevent crevice corrosion by minimizing tight spaces in the design of the object. Crevice corrosion occurs in spaces of a metal object where access to the surrounding fluid (air or a liquid) is poor - for instance, under screws, under washers, under barnacles, or between the joints of a hinge. Crevice corrosion occurs where the gap near a metal surface is wide enough to allow fluid to enter but narrow enough that the fluid has difficulty leaving and becomes stagnant. The local environment in these small spaces becomes corrosive and the metal begins to corrode in a process similar to pitting corrosion. Preventing crevice corrosion is generally a design issue. By minimizing the occurrence of tight gaps in a metal object's construction through closing these gaps or allowing circulation, it's possible to minimize crevice corrosion.
- Crevice corrosion is of special concern when dealing with metals like aluminum which have a protective, passive outer layer, as the mechanism of crevice corrosion can contribute to the breakdown of this layer.
-
5Prevent stress corrosion cracking by using only safe loads and/or annealing. Stress corrosion cracking (SCC) is a rare form of corrosion-related structural failure that is of particular concern to engineers charged with building structures intended to support important loads. In the event of SCC, a load-bearing metal forms cracks and fractures below its specified load limit - in severe cases, at a fraction of the limit. In the presence of corrosive ions, tiny, microscopic cracks in the metal caused by tensile stress from a heavy load propagate as the corrosive ions reach the tip of the crack. This causes the crack to gradually grow and potentially cause eventual structural failure. SCC is especially dangerous because it can occur even in the presence of substances that are naturally only very mildly corrosive to the metal. This means that the dangerous corrosion occurs while the rest of the metal surface superficially appears unaffected.
- Preventing SCC is partly a design issue. For instance, by choosing a material that is SCC-resistant in the environment in which the metal will operate and ensuring that the metal material is properly stress-tested can help prevent SCC. Additionally, the process of annealing a metal can eliminate residual stresses from its manufacture.
- SCC is known to be exacerbated by high temperatures and the presence of liquid containing dissolved chlorides.
Preventing Corrosion with Home Solutions
-
1Paint the metal surface. Perhaps the most common, affordable method of protecting metal from corrosion is simply to cover it up with a layer of paint. The process of corrosion involves moisture and an oxidizing agent interacting with the surface of the metal. Thus, when the metal is coated with a protective barrier of paint, neither moisture nor oxidizing agents can come into contact with the metal itself and no corrosion occurs.
- However, paint itself is vulnerable to degradation. Reapply paint whenever it becomes chipped, worn or damaged. If paint degrades to the point that the underlying metal becomes exposed, be sure to inspect for corrosion or damage on the exposed metal.
- There are a variety of methods for applying paint to metal surfaces. Metalworkers often use several of these methods in conjunction to ensure that the entire metal object receives a thorough coating. Below is a sampling of methods with comments on their usages:
- Brush - used for hard-to-reach spaces.
- Roller - used for covering large areas. Cheap and convenient.
- Air spray - used for covering large areas. Quicker but less efficient than rollers (paint wastage is high).
- Airless spray/Electrostatic airless spray - used for covering large areas. Quick and allows for variable levels of thick/thin consistency. Less wasteful than ordinary air spray. Equipment is expensive.
-
2Use marine paint for metal exposed to water. Metal objects that regularly (or constantly) come into contact with the water, like boats, require special paints to protect against the increased possibility of corrosion. In these situations, "normal" corrosion in the form of rusting isn't the only concern (though it is a major one), as marine life (barnacles, etc.) that can grow on unprotected metal can become an additional source of wear and corrosion. To protect metal objects like boats and so on, be sure to use a high-grade marine epoxy paint. Not only do these types of paint protect the underlying metal from moisture, but also discourage the growth of marine life on its surface.
-
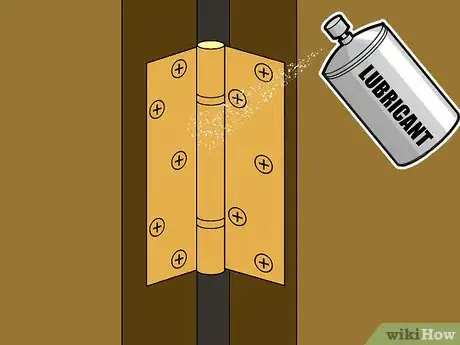
3Apply protective lubricants to moving metal parts. For flat, static metal surfaces, paint does a great job of keeping out moisture and preventing corrosion without affecting the metal's usefulness. However, paint usually isn't suitable for moving metal parts. For instance, if you paint over a door hinge, when the paint dries, it will hold the hinge in place, hindering its motion. If you force the door open, the paint will crack, leaving holes for moisture to reach the metal. A better choice for metal parts like hinges, joints, bearings, and so on is a suitable water-insoluble lubricant. A thorough coat of this type of lubricant will naturally repel moisture while simultaneously ensuring the smooth, easy motion of your metal part.
- Because lubricants don't dry in place like paints, they degrade over time and require occasional re-application. Reapply lubricants to metal parts periodically to ensure they remain effective as protective sealants.
-
4Clean metal surfaces thoroughly before painting or lubricating. Whether you're using normal paint, marine paint, or a protective lubricant/sealant, you'll want to ensure your metal is clean and dry before starting the application process. Take care to ensure the metal is entirely free of dirt, grease, residual welding debris, or existing corrosion, as these things can undermine your efforts by contributing to future corrosion.
- Dirt, grime, and other debris interferes with paint and lubricants by keeping the paint or lubricant from adhering directly to the metal surface. For instance, if you paint over a sheet of steel with a few stray metal shavings on it, the paint will set on the shavings, leaving blank spaces on the underlying metal. If and when the shavings fall off, the exposed spot will be vulnerable to corrosion.
- If painting or lubricating a metal surface with some existing corrosion, your goal should be to make the surface as smooth and regular as possible to ensure the best possible adherence of the sealant to the metal. Use a wire brush, sandpaper, and/or chemical rust removers to remove as much loose corrosion as possible.
-
5Keep unprotected metal products away from moisture. As noted above, most forms of corrosion are exacerbated by moisture. If you can't manage to give your metal a protective coating of paint or sealant, you should take care to ensure it's not exposed to moisture. Making an effort to keep unprotected metal tools dry can improve their usefulness and lengthen their effective life.If your metal items are exposed to water or moisture, be sure to clean and dry them immediately following use to prevent corrosion from starting.
- In addition to watching for exposure to moisture during use, be sure to store the metal items indoors in a clean, dry place. For large objects that won't fit in a cupboard or closet, cover the object with a tarp or cloth. This helps keep out moisture from the air and prevents dust from accumulating on the surface.
-
6Keep metal surfaces as clean as possible. After each use of a metal item, whether the metal is painted or not, be sure to clean its functional surfaces, removing any dirt, grime, or dust. Accumulations of dirt and debris on metal surface can contribute to the wear and tear of the metal and/or its protective coating, leading to corrosion over time.
Preventing Corrosion with Advanced Electrochemical Solutions
-
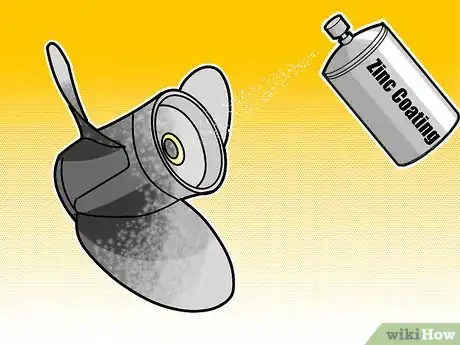
1Use a galvanization process. Galvanized metal is metal that has been coated with a thin layer of zinc to protect it from corrosion. Zinc is more chemically-active than the underlying metal[7] , so it oxidizes when exposed to air. Once the zinc layer oxidizes, it forms a protective coating, preventing further corrosion of the metal underneath. The most common type of galvanization today is a process called hot-dip galvanization in which metal parts (usually steel) are submerged in a vat of hot, molten zinc to gain a uniform coating.
- This process involves handling industrial chemicals, some of which are hazardous at room temperature, at extremely hot temperatures and thus should not be attempted by anyone other than trained professionals. Below are the basic steps of the hot-dip galvanization process for steel:
- The steel is cleaned with a caustic solution to remove dirt, grease, paint, etc., then thoroughly rinsed.
- The steel is pickled in acid to remove mill scale, then rinsed.
- A material called a flux is applied to the steel and allowed to dry. This helps the final zinc coating adhere to the steel.
- The steel is dipped in a vat of molten zinc and allowed to heat to the temperature of the zinc.
- The steel is cooled in a "quench tank" containing water.
- This process involves handling industrial chemicals, some of which are hazardous at room temperature, at extremely hot temperatures and thus should not be attempted by anyone other than trained professionals. Below are the basic steps of the hot-dip galvanization process for steel:
-
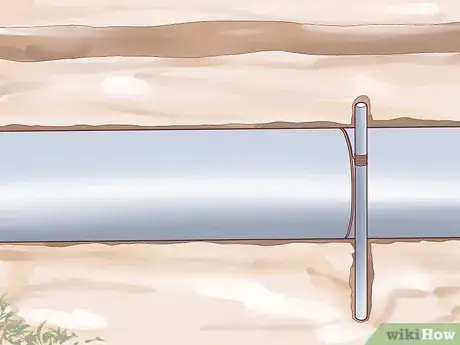
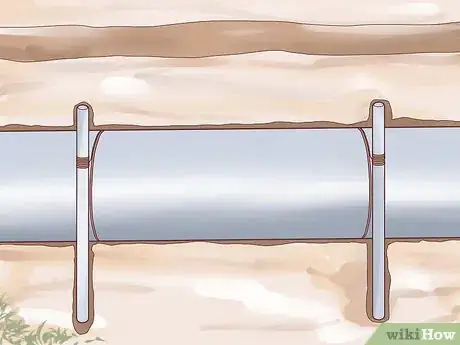
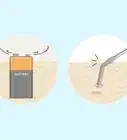
2Use a sacrificial anode. One way to protect a metal object from corrosion is to electrically attach a small, reactive piece of metal called a sacrificial anode to it. Because of the electrochemical relationship between the larger metal object and the small reactive object (explained briefly below), only the small, reactive piece of metal will undergo corrosion, leaving the large, important metal object intact. When the sacrificial anode corrodes completely, it must be replaced or the larger metal object will begin to corrode. This method of corrosion protection is often used for buried structures, like underground storage tanks, or objects in constant contact with water, like boats.
- Sacrificial anodes are made from several different types of reactive metal. Zinc, aluminum, and magnesium are three of the most common metals used for this purpose. Because of the chemical properties of these materials, zinc and aluminum are often used for metal objects in saltwater, whereas magnesium is more suitable for fresh water purposes.
- The reason a sacrificial anode works has to do with the chemistry of the corrosion process itself. When a metal object corrodes, areas that chemically resemble the anodes and cathodes in an electrochemical cell naturally form. Electrons flow from the most anode parts of the metal surface into surrounding electrolytes. Since sacrificial anodes are very reactive compared to the metal of the object being protected, the object itself becomes very cathodic in comparison and, thus, electrons flow out of the sacrificial anode, causing it to corrode but sparing the rest of the metal.
-
3Use impressed current. Because the chemical process behind metal corrosion involves electric current in the form of electrons flowing out of the metal, it's possible to use an outside source of electric current to overpower the corrosive current and prevent corrosion. Essentially, this process (called impressed current) confers a continuous negative electrical charge on the metal being protected. This charge overpowers the current causing electrons to flow out of the metal, halting corrosion. This type of protection is often used for buried metal structures like storage tanks and pipelines.
- Note that the type of current used for impressed current protection systems is usually direct current (DC).
- Usually, corrosion-preventing impressed current is generated by burying two metal anodes in the soil near the metal object to be protected. Current is sent through an insulated wire to the anodes, which then flows through the soil and into the metal object. Current passes through the metal object and returns to the source of the current (generator, rectifier, etc.) through an insulated wire. [8]
-
4Use anodization. Anodizing is a special type of protective surface coating used to protect metal from corrosion and also to apply to apply dies and so forth. If you've ever seen a brightly-colored metal carabiner, you've seen a dyed anodized metal surface. Rather than involving the physical application of a protective coating, as with painting, anodizing uses an electric current to give the metal a protective coating that prevents nearly all forms of corrosion.
- The chemical process behind anodization involves the fact that many metals, like aluminum, naturally form chemical products called oxides when they come into contact with oxygen in the air. This results in the metal normally having a thin outer oxide layer which protects (to varying degree, depending on the metal) against further corrosion. The electric current used in the anodizing process essentially creates a much thicker buildup of this oxide on the surface of the metal than would normally occur, providing great protection from corrosion.
- There are several different ways to anodize metals. Below are the basic steps of one anodizing process.[9]
See How to Anodize Aluminum for more information.
- The aluminum is cleaned and de-greased.
- The aluminum's surface impurities are removed with a de-smut solution.
- The aluminum is lowered into an acid bath at a constant current and temperature (for instance, 12 amps/sq ft and 70-72 degrees F (21-22 degrees C).
- The aluminum is removed and rinsed.
- The aluminum is optionally submerged in dye at 100-140 degrees F (38-60 degrees C).
- The aluminum is sealed by placing it in boiling water for 20-30 minutes.
-
5Use a metal that exhibits passivation. As noted above, some metals naturally form a protective oxide coating upon exposure to air. Some metals form this oxide coating so effectively that they eventually become relatively chemically inactive. We say that these metals are passive in reference to the process of passivation by which they become less reactive. Depending on its desired use, a passive metal object may not necessarily need any extra protection to make it corrosion-resistant.
- One well-known example of a metal that exhibits passivation is stainless steel. Stainless steel is an alloy of ordinary steel and chromium that is effectively corrosion-proof in most conditions without requiring any other protection. For most day-to-day uses, corrosion isn't usually a concern with stainless steel.
- However, it bears mentioning that in certain conditions, stainless steel is not 100% corrosion-proof - notably, in salt water. Similarly, many passive metals become non-passive under certain extreme conditions and thus may not be suited for all uses.
- One well-known example of a metal that exhibits passivation is stainless steel. Stainless steel is an alloy of ordinary steel and chromium that is effectively corrosion-proof in most conditions without requiring any other protection. For most day-to-day uses, corrosion isn't usually a concern with stainless steel.
Expert Q&A
-
QuestionHow can you prevent polished aluminum from tarnishing?
 Cleanzen Cleaning ServicesThe Cleanzen Cleaning Services Team consists of Residential Cleaning Specialists. With more than six years of experience, they specialize in connecting independent cleaning professionals with those who need help cleaning their houses. All of Cleanzen’s professionals are experienced and licensed and have passed background checks.
Cleanzen Cleaning ServicesThe Cleanzen Cleaning Services Team consists of Residential Cleaning Specialists. With more than six years of experience, they specialize in connecting independent cleaning professionals with those who need help cleaning their houses. All of Cleanzen’s professionals are experienced and licensed and have passed background checks.
Residential Cleaning Specialists Polished aluminum is prone to tarnishing because of oxidation. To prevent oxidation and corrosion, apply a special anodic coating material. These are available at local hardware and home improvement stores in the paints and coatings section.
Polished aluminum is prone to tarnishing because of oxidation. To prevent oxidation and corrosion, apply a special anodic coating material. These are available at local hardware and home improvement stores in the paints and coatings section. -
QuestionHow does oxygen affect metals?
 wikiHow ContributorCommunity AnswerThrough oxidation. Fe + O2 = Fe Oxide. Fe is Iron, and this rusts the metal, making it brownish yellow at times, and removes the advantages of using it.
wikiHow ContributorCommunity AnswerThrough oxidation. Fe + O2 = Fe Oxide. Fe is Iron, and this rusts the metal, making it brownish yellow at times, and removes the advantages of using it.
References
- ↑ http://www.estainlesssteel.com/corrosion.shtml
- ↑ http://corrosion-doctors.org/MatSelect/corrbrass.htm
- ↑ http://www.aluminiumdesign.net/design-support/aluminium-corrosion-resistance/
- ↑ http://www.aluminiumdesign.net/design-support/aluminium-corrosion-resistance/
- ↑ http://corrosion-doctors.org/Definitions/galvanic-series.htm
- ↑ http://www.nace.org/General-Attack-Corrosion/
- ↑ https://www.boundless.com/chemistry/electrochemistry/corrosion/preventing-corrosion/
- ↑ http://www.epa.gov/oust/ustsystm/cathodic.htm
- ↑ http://bryanpryor.com/anodizing.php
About This Article
To prevent metals from corroding, keep unprotected metals away from moisture by storing them in a dry place, like a cupboard. If they can’t fit indoors, cover them with a tarp. To protect metal, start by cleaning the surface of any dirt, debris or grease. Then, coat the area in a layer of paint or an oil sealant. If your metal comes in contact with water, like a boat, use a marine paint to keep it protected. Alternatively, cover moving surfaces, like door hinges, with a water-soluble lubricant. For more ways to prevent metals from corroding, like halting ion flow between metals or avoiding nicks and scratches, read on!